Abstract
Objective: We used the KINARM robot to quantify impairments in cognitive and upper-limb sensorimotor performance in a cohort of people with amyotrophic lateral sclerosis (ALS). We sought to study the feasibility of using this technology for ALS research, to quantify patterns of impairments in individuals living with ALS, and elucidate correlations between robotic and traditional clinical behavioral measures. Methods: Participants completed robot-based behavioral tasks testing sensorimotor, cognitive, and proprioceptive performance. Performance on robotic tasks was normalized to a large healthy control cohort (no neurological impairments), adjusted for age. Task impairment was defined as performance outside the 95% range of controls. Traditional clinical tests included: Frontal Assessment Battery (FAB), ALS Functional Rating Scale-Revised (ALSFRS-R), and Montreal Cognitive Assessment (MoCA). Results: Seventeen people with ALS were assessed. Two participants reported pain or discomfort from the robot’s seat and 2 others reported discomfort from arm position during the assessment (both rectified and did not affect exam completion). Participants were able to perform the majority of the robotic tasks, although 9 participants were unable to complete 1 or more tasks. Between 20 and 69% of participants displayed sensorimotor impairments; 19 and 69% displayed cognitive task impairments; 25% displayed proprioceptive impairments. MoCA was impaired in 9/17 participants; 10/17 had impaired performance on FAB. MoCA and FAB correlated well with robot-based measures of cognition. Conclusion: Use of robotic assessment is generally feasible for people with ALS. Individuals with ALS have sensorimotor impairments as expected, and some demonstrate substantial cognitive impairments.
Introduction
Amyotrophic lateral sclerosis (ALS) is primarily considered as a disease of motor neurons and, as such, clinical assessment of ALS is usually achieved with a combination of EMG as well as pen-and-paper tools to quantify motor dysfunction. One of the traditional pen-and-paper tools used to quantify motor functionality in ALS is the ALS Functional Rating Scale-Revised (ALSFRS-R) [Citation1]. The ALSFRS-R captures the ability to perform daily life activities, scoring each of 15 items on a 0–4 point scale [Citation1]. This scale captures several aspects of daily living (e.g. dressing oneself and writing), but each item is subjectively scored and the measurements are coarse. Subtle aspects of performance on each item, such the quantity of time required to complete them or the precision with which they are completed, cannot be appreciated using the ALSFRS-R. Thus, assessments of motor performance in ALS are needed that encompass multiple aspects of motor behavior and are quantitative.
While progressive motor weakness is a major contributor to disability in ALS, it is becoming increasingly recognized that cognitive decline also plays a significant role. Cognitive deficits in people with ALS have been characterized previously [Citation2], including impairments in response inhibition [Citation3], set-switching [Citation4], and spatial working memory [Citation5]. Individuals with early cognitive deficits experience a more rapid functional decline than those without cognitive deficits [Citation6]. Therefore, objective measurement of cognitive deficits in ALS is valuable for gauging disease progression, easing caregiver burden, and may permit more timely diagnosis, allowing earlier treatment initiation.
Here, we study the feasibility of using robotic technology (the KINARM Exoskeleton lab; BKIN Technologies, Kingston, ON, Canada) to assess motor behavior in people with ALS. KINARM Standard Tests were initially developed and tested on individuals with stroke to quantify impairments in upper limb sensory, motor and cognitive functions [Citation7–15]. These tasks have also been used to quantify impairments associated with concussion [Citation16], traumatic brain injury [Citation17], cerebral palsy [Citation18,Citation19] and transient ischemic attack [Citation14]. In the present study, participants performed a battery of 9 behavioral tasks that assess upper limb motor and proprioceptive performance, as well as aspects of cognitive function. We also performed a series of traditional clinical assessments to act as a benchmark for measuring behavioral characteristics in this population. We hypothesize that (1) KINARM testing will be feasible for people with the heterogeneous presentation of ALS, (2) people with ALS will display deficits on tasks assessing sensory, motor, and cognitive performance, and (3) we will observe correlations between clinical tests and robotic assessment tasks.
Methods
Participants and clinical assessment
Participants were recruited from the Adult Neuromuscular Disease clinic at Kingston General Hospital and Providence Care Hospital (both Kingston, ON, Canada). Inclusion criteria were: (1) diagnosis of probable/definite ALS based on El Escorial criteria [Citation20] with/without frontotemporal dementia (FTD), and (2) the ability to understand clinical and robotic assessment instructions. People with primary lateral sclerosis (PLS) were also accepted. Lower/upper motor neuron or bulbar etiology at initial diagnosis was determined by an experienced neurologist (ST). The local Ethics Review Boards reviewed and approved this study, and participants provided written informed consent prior to taking part.
We performed five clinical assessments: (1) Frontal Assessment Battery (FAB) [Citation21], (2) Montreal Cognitive Assessment (MoCA) [Citation22], (3) ALS Functional Rating Scale-Revised (ALSFRS-R) [Citation1], (4) Purdue pegboard [Citation23], and (5) assessment of upper limb strength. FAB assesses frontal lobe executive function using six individual tasks targeting different aspects of cognition and has been used previously on individuals with ALS [Citation24]. FAB was chosen for its speed of administration and reliability in assessing frontal lobe function in people with ALS [Citation25]. Impaired performance on the FAB was a score <18. MoCA is used as a screen for mild cognitive impairment, with healthy performance ≥26 [Citation22]. ALSFRS-R consists of 4 domains with 15 sub-scores each worth 4 points. The score for each domain was composed of [domain (sub-scores)]: Fine motor (writing, feeding, turning, dressing), gross motor (turning, dressing, walking, climbing, dyspnea), respiration (swallowing, orthopnea, dyspnea, respiratory insufficiency), and bulbar (speech, swallowing, salivation, orthopnea) [Citation1]. Impairment on each domain is indicated by a sub-score <16 (fine motor), <20 (gross motor), <16 (respiration), or <16 (bulbar), respectively. Overall impairment on the ALSFRS-R is indicated by a score <48. The Purdue pegboard assessment measures the number of pegs that can be inserted into a board in 30 seconds [Citation23]. Finally, strength was quantified in the right and left deltoids, biceps, and triceps muscles, on a scale of 1–5 (5 indicates normal strength).
Robotic assessment
Participants performed 9 behavioral tasks using the KINARM Exoskeleton Classic lab (BKIN Technologies, Kingston, ON). Participants sat in the robotic exoskeleton with their arms placed in troughs attached to the mechanical linkage that permitted flexor and extensor motion of the shoulder and elbow joints in the horizontal workspace. A virtual reality system provided visual feedback of movement on a screen aligned with the horizontal workspace of the arm. Visual feedback of the participant’s hand was obscured with a physical barrier (). Additional lumbar support was provided to the chair of the robot to assist with posture/provide additional comfort.
Figure 1 The KINARM exoskeleton robot and robotic assessment tasks. (a) The KINARM exoskeleton robot allows patients to sit and perform behavioral tasks as weight support is provided to their arms. Tasks are displayed on a horizontal virtual reality display aligned with the horizontal workspace of the arm. (b) Tasks are arranged in cognitive, motor, and sensory groups. Motor tasks: clockwise from Ball on Bar to Elbow Stretch (ND). Sensory task: Arm Position Matching. Cognitive tasks: clockwise from Spatial Span to Reverse Visually-Guided Reaching (inter-limb). Small markers: Task Scores above 1.96 indicate impairment as they are outside the 95th percentile of performance of healthy subjects. Large markers: performance within the 95th percentile of healthy controls.
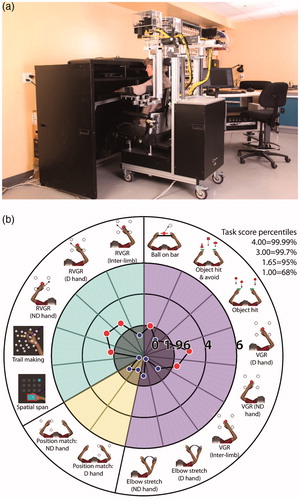
Five tasks assessed upper limb sensorimotor function. These included visually guided reaching (VGR) [Citation8,Citation13], object hit (OH) [Citation9], object hit-and-avoid (OHA) [Citation10], ball-on-bar (BOB) [Citation12], and elbow stretch test (EST) [Citation11]. One task was used to assess upper limb proprioceptive function, which was arm position matching (APM) [Citation7]. Finally, three tasks assessed cognition. These were reverse visually guided reaching (RVGR) [Citation14,Citation26], spatial span (SPS) [Citation27], and trail-making (TMT) [Citation15,Citation28]. For RVGR and VGR, inter-limb performance was also assessed by subtracting (dominant arm performance)-(nondominant arm performance) prior to statistical analysis, to quantify impairment laterality in these tasks. For task descriptions and associated citations, see (or www.bkintechnologies.com).
Table 1 Task descriptions.
Statistical analysis
Performance in each task was quantified using multiple parameters that captured the spatial and temporal features of behavior. We quantified a Task Score that reflects the global performance of an individual on a given task normalized to a population of healthy control participants (n = 94–494, depending on the task). We have described this process elsewhere [Citation14] (also www.bkintechnologies.com). Briefly, healthy participant performances for each task parameter were transformed into normal distributions. These distributions were de-skewed using Box-Cox equations [Citation29]. Linear regressions were performed to identify the effects of age. The normality of these distributions was tested and the Box-Cox equations were adjusted to attain normality as required. Outliers were removed at this point if necessary (Z-scores>|3.29|). The process of de-skewing and outlier removal was iterated up to 3 times.
A Task Score was generated using the root-mean-square (RMS) of the Z-scores for all task parameters that could be normalized. RMS values were themselves normalized using Box-Cox transforms, linear regressions to quantify the influence of noise, and finally outlier removal, similar to above. This distribution of RMS-Z scores was then converted to an exclusively-positive measure. Task Score distribution percentiles approximate those of the standard Normal distribution (). A Task Score has similar properties to a Z-score, and therefore a value of >1.96 reflects performance outside 95% of performances of healthy controls (considered to be impaired).
Finally, we used Pearson correlations to compare robotic tasks and clinical measures. We corrected for multiple comparisons by dividing the standard 2-tailed p = 0.05 by 15 (the number of clinical or robotic variables) to yield a new significant correlation p value of 0.0033.
Results
Demographics and clinical assessment
Seventeen people with ALS participated. The average age was 64.4 ± 7.7 years. Twelve had diagnoses of definite ALS, 4 had diagnoses of probable ALS, and 1 had a diagnosis of PLS. One participant (P14) also had FTD. Etiologies of ALS at initial diagnosis prior to our study were lower motor neuron dysfunction in 12 participants, upper motor neuron dysfunction in 4 participants, and bulbar dysfunction in 1 participant. All participants were right-handed and 13/17 were male (76%).
Many participants showed poor performance in traditional clinical tests assessing cognition and daily life functionality. MoCA was abnormal in 9/17 participants (53%) and the (average ± SD) score was 25.5 ± 3.5. FAB was impaired in 10/17 (59%) participants; average ± SD score was 15.7 ± 3.0. All participants had abnormal ALSFRS-R total scores (34.8 ± 5.2). All participants showed impairment on gross motor and fine motor components of the ALSFRS-R. Respiratory function and bulbar function were impaired in 8 and 14 participants, respectively. Detailed data are presented in .
Table 2 Individual-specific clinical and demographic data.
Robotic assessment
We tested 17 people with ALS using the robotic system. Two reported feeling pain or discomfort from the robot’s seat, and 2 others reported discomfort due to arm positions required by certain tasks (pain/discomfort was immediately rectified). Most participants (12/17) required a harness to assist in maintaining an upright posture for the duration of the assessment.
Nine participants were unable to complete 1 or more tasks (15 total instances of uncompleted tasks, i.e. some did not finish multiple tasks). Reasons that tasks were not completed included fatigue, unable to initiate the task, not attempting the task, or not understanding the task. The most common reasons were that the task was not attempted (9 instances) or not understood (3 instances). Participant P15 was unable to complete most tasks, finishing only VGR and RVGR ().
Table 3 Individual task scores for all participants in the study.
Individuals with ALS often displayed performance significantly below the average of healthy controls in robot-based tasks (). As expected, most had impairments in motor-related tasks. Most commonly, motor-related impairments were found in OH. Fifty-six percent (9/16) showed impairments in OH. In EST, 77% (10/13) of participants showed impairment in either arm which included 57% (8/14) in the dominant arm and 64% (9/14) in the non-dominant arm (some were impaired bilaterally). In VGR, 59% (10/17) of participants showed impairments in either arm (some were impaired in both arms). This included 59% (10/17) in VGR-dominant arm and 27% (4/15) in VGR non-dominant arm, respectively. Twenty-percent (3/15) fell outside of the expected range of inter-limb differences. Fifty percent (8/16) and 36% (4/11) showed impairments on BOB and OHA, respectively.
In addition to motor impairments, participants frequently displayed cognitive and sensory impairments (). The most common was in RVGR: 11/16 (69%) were impaired on this task in either arm including 69% (11/16) in the dominant arm and 53% (8/15) in the non-dominant arm, respectively. The inter-limb difference was outside the normal range in 60% (9/15) of participants. TMT and SPS impairments were found in 19% (3/16) and 25% of participants, respectively. Finally, APM performance was impaired in 25% (4/16) of participants in either arm; 19% (3/16) in the dominant arm and 6% (1/16) in the non-dominant arm.
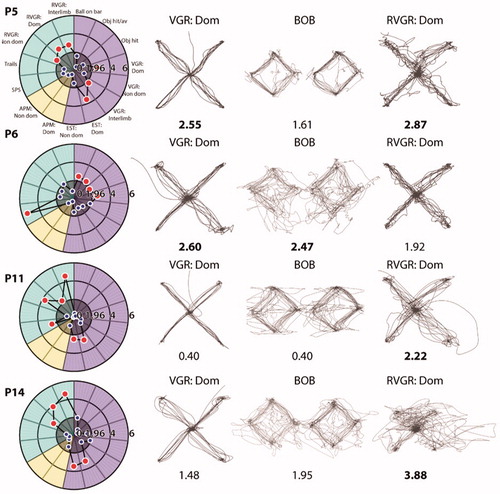
There was considerable variability in impairment patterns across tasks (, ). Poor performance could be observed across predominantly motor tasks, as exemplified by participant P6. In contrast, participant P11 was impaired often in cognitive tasks. Finally, participants P5 and P14 were impaired in RVGR and either VGR or EST. additionally provides depictions of performance on 2 motor tasks (VGR and BOB) and 1 cognitive task (RVGR).
Figure 2 Example patient performance on robotic assessment tasks. Each row displays Task Scores for four example participants and hand paths for three tasks. Task Scores are written underneath each task and are indicated as impaired (bold) when appropriate. ‘Dom’ denotes dominant hand and ‘Non-dom’ denotes non-dominant hand.
Finally, we investigated the correlation between robotic tasks and clinical scores. After multiple comparisons correction, this number reduced to 4. These significant correlations were between MoCA and SPS (R= −0.71; p < 0.0033); MoCA and TMT (R= −0.72; p < 0.0033); FAB and SPS (R= −0.88; p < 0.0033); FAB and TMT (R= −0.75; p < 0.0033). See .
Table 4 Correlations between clinical scores and robotic tasks.
Discussion
We examined the use of robot-based behavioral tasks to quantify sensory, motor, and cognitive impairments in a small cohort of individuals with ALS. Most people with ALS completed all behavioral tasks, demonstrating the potential feasibility of using robotic assessment in this population. Impairments varied substantially across participants, but commonly included motor and cognitive tasks.
While most participants with ALS completed the majority of behavioral tasks, there were some obstacles to using this technology with this population that would have to be addressed to increase its general utility for assessing individuals with ALS. Several participants were unable to complete tasks for reasons including: inability to start the task, not attempting the task, fatigue, and not understanding task instructions. Most commonly, tasks were not attempted. Fatigue [Citation30] and/or low motivation [Citation31] may have contributed, which are common in ALS. Fatigue was addressed by using an exoskeleton robot to support the upper limbs’ weight and a harness to aid posture; thus, even participants with severe weakness could complete most tasks. Future studies may further mitigate fatigue by implementing a task hierarchy to reduce the number of tasks performed [Citation32]. Inability to persevere through tasks could be mitigated by re-designing tasks to be more interesting, encouraging participation. Some participants did not complete tasks because of difficulty understanding task requirements, likely reflecting cognitive impairments associated with ALS. Finally, a key limitation of some of the tasks that we employed in this study is that they were developed for stroke patients who predominantly have impairments localized to one side (contralateral to the lesion). ALS often presents bilaterally, and thus many tasks quantifying laterality (e.g. OH) may require redesign to better-capture performance deficits.
In spite of these challenges, we collected data from most participants and, as expected, many participants had motor impairments. Motor impairments were commonly found in tasks requiring predominantly speed (OH) and accuracy (VGR). After multiple-comparisons correction, no correlations between clinical and robotic motor tests were significant. However, many of them had magnitudes >|0.70|, indicating potentially strong relationships. These correlations seem reasonable. For example, BOB performance correlates to both right arm strength and Purdue pegboard performance (all quantify speed/strength or accuracy). Missing data for some tests, such as the Purdue pegboard, may have affected these correlations in our small sample. Conversely, tests such as the ALSFRS-R may not be expected to correlate well with robotic measures as ALSFRS-R sub-scores do not quantify speed or accuracy, unlike our robotic system.
Cognition was assessed using three tasks: RVGR, SPS, and TMT. Sixty-nine percent of participants were impaired on RVGR, which required inhibiting the automatic response to reach towards a visual goal and instead reach in the opposite direction towards a spatial goal [Citation33,Citation34]. Several participants that were impaired on RVGR were not impaired in VGR, demonstrating a distinct impairment in cognitive-motor integration and not motor performance on its own. Given that RVGR requires inhibition of a motor response, this may reflect a deficit in inhibition which is known to exist in ALS [Citation25,Citation33]. SPS and TMT were each impaired in 20–25% of participants, possibly reflecting that some people with ALS may have both memory- and processing speed deficits [Citation2,Citation34]. The good correlations between cognitive robotic- and clinical tests suggest that these tasks are capable of assessing cognition independently of motor performance. Separation of cognitive and motor deficits in ALS may also be facilitated by measuring eye movements, however, eye movements can also be affected in ALS [Citation35,Citation36]. It will be interesting in the future to correlate robotic motor task findings with disease stage and neuroimaging. The generalizability of these findings should be improved in future work, given our small sample size and the heterogeneity of our cohort. Greater focus on a single clinical endotype may shed more light on the issue of generalizing findings to the broader ALS population.
Conclusion
We report that robotic testing is generally feasible for assessing individuals with ALS in our small cohort, which may be promising for the feasibility of robotic assessment in the ALS population. We have corroborated findings from the literature with respect to impairments in multiple cognitive domains, and have provided novel evidence of deficits in motor function and cognitive-motor integration. This supports the notion that executive and motor planning dysfunction is present in people with ALS and that it extends beyond motoneurons and the corticospinal tract. Several robot-based tasks are not easily measured using standard neurological testing, and we highlight the potential of robotic assessment in providing additional information that may be useful for therapeutic decision-making and clinical trials.
Declaration of interest
SHS is the co-founder and Chief Scientific Officer of BKIN Technologies Ltd., the company that commercializes the KINARM robot.
Acknowledgements
We would like to thank Simone Appaqaq and Kim Moore for their assistance with scheduling and examining participants. We would like to thank Helen Bretzke and Justin Peterson for their technical assistance with the KINARM robot and data management.
Data availability
All relevant data are included in the manuscript, figures, and tables.
Additional information
Funding
References
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21.
- Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6:994–1003.
- Goldstein LH, Newsom-Davis IC, Bryant V, Brammer M, Leigh PN, Simmons A. Altered patterns of cortical activation in ALS patients during attention and cognitive response inhibition tasks. J Neurol. 2011;258:2186–98.
- Lange F, Vogts M, Seer C, Fürkötter S, Abdulla S, Dengler R, et al. Impaired set-shifting in amyotrophic lateral sclerosis: an event-related potential study of executive function. 2016;30:120–34.
- Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65:586–90.
- Elamin M, Bede P, Byrne S, Jordan N, Gallagher L, Wynne B, et al. Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology. 2013;80:1590–7.
- Dukelow SP, Herter TM, Moore KD, Demers MJ, Glasgow JI, Bagg SD, et al. Quantitative assessment of limb postion sense following stroke. Neurorehabil Neural Repair. 2010;24:178–87.
- Coderre AM, Abou Zeid A, Dukelow SP, Demmer MJ, Moore KD, Demers MJ, et al. Assessment of upper-limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabil Neural Repair. 2010;24:528–41.
- Tyryshkin K, Coderre AM, Glasgow JI, Herter TM, Bagg SD, Dukelow SP, et al. A robotic object hitting task to quantify sensorimotor impairments in participants with stroke. J NeuroEngineering Rehabil. 2014;11:47.
- Bourke TC, Lowrey CR, Dukelow SP, Bagg SD, Norman KE, Scott SH. A robot-based behavioural task to quantify impairments in rapid motor decisions and actions after stroke. J Neuroeng Rehabil. 2016;13:91.
- Centen A, Lowrey CR, Scott SH, Yeh TT, Mochizuki G. KAPS (kinematic assessment of passive stretch): a tool to assess elbow flexor and extensor spasticity after stroke using a robotic exoskeleton. J Neuroeng Rehab. 2017;14:1–13.
- Lowrey CR, Jackson CPT, Bagg SD, Dukelow SP, Scott SH. A novel robotic task for assessing impairments in bimanual coordination post-stroke. Int J Phys Med Rehabil. 2014;S3:1–10.
- Mostafavi SM, Dukelow SP, Glasgow JI, Scott SH, Mousavi P. Reduction of stroke assessment time for visually guided reaching task on KINARM exoskeleton robot. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5296–9.
- Simmatis L, Krett J, Scott SH, Jin AY. Robotic exoskeleton assessment of transient ischemic attack. PLoS One. 2017;12:1–13.
- Little CE, Emery C, Black A, Scott SH, Meeuwisse W, Nettel-Aguirre A, et al. Test-retest reliability of KINARM robot sensorimotor and cognitive assessment: in pediatric ice hockey players. J Neuroeng Rehab. 2015;12:78.
- Subbian V, Ratcliff JJ, Korfhagen JJ, Hart KW, Meunier JM, Shaw GJ, et al. A novel tool for evaluation of mild traumatic brain injury patients in the emergency department: does robotic assessment of neuromotor performance following injury predict the presence of postconcussion symptoms at follow-up?. Acad Emerg Med. 2016;23:382–92.
- Debert CT, Herter TM, Scott SH, Dukelow S. Robotic assessment of sensorimotor deficits after traumatic brain injury. J Neurol Phys Ther. 2012;36:58–67.
- Kuczynski AM, Semrau JA, Kirton A, Dukelow SP. Kinesthetic deficits after perinatal stroke: robotic measurement in hemiparetic children. J Neuroeng Rehab. 2017;14:1–14.
- Kuczynski AM, Dukelow SP, Hodge JA, Carlson HL, Lebel C, Semrau JA, et al. Corticospinal tract diffusion properties and robotic visually guided reaching in children with hemiparetic cerebral palsy. Hum Brain Mapp. 2018;39:1130–44.
- Brooks BR, Miller RG, Swash M, Munsat TL, Rix B, Miller RG, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. 2000;1:293–9.
- Dubois B, Slachevsky a, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–6.
- Nasreddine ZS, Phillips NA, BÃcdirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9.
- Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–47.
- Seer C, Fürkötter S, Vogts M-B, Lange F, Abdulla S, Dengler R, et al. Executive dysfunctions and event-related brain potentials in patients with amyotrophic lateral sclerosis. Front Aging Neurosci. 2015;7:225.
- Ahn SW, Kim SH, Kim JE, Kim SM, Kim SH, Sung JJ, et al. Frontal assessment battery to evaluate frontal lobe dysfunction in ALS patients. Can J Neurol Sci. 2011;38:242–6.
- Hawkins KM, Sergio LE. Visuomotor impairments in older adults at increased Alzheimer’s disease risk. Jad. 2014;42:607–21.
- Corsi PM. Memory and the medial temporal region of the brain [dissertation]. Montreal (Quebec): McGill University; 1972.
- Arbuthnott K, Frank J. Trail making test, part b as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–28.
- Box GE. Cox D. An analysis of transformations. J R Stat Soc Ser B. 1964;26:211–52.
- Ramirez C, Piemonte MEP, Callegaro D, Da Silva HCA. Fatigue in amyotrophic lateral sclerosis: frequency and associated factors. Amyotroph Lateral Scler. 2008;9:75–80.
- Lillo P, Mioshi E, Zoing MC, Kiernan MC, Hodges JR. How common are behavioural changes in amyotrophic lateral sclerosis?. Amyotroph Lateral Scler. 2011;12:45–51.
- Mostafavi SM, Scott S, Dukelow S, Mousavi P. Reduction of assessment time for stroke-related impairments using robotic evaluation. IEEE Trans Neural Syst Rehabil Eng. 2017;25:945–55.
- Olney RK, Murphy J, Forshew D, Garwood E, Miller BL, Langmore S, et al. The effects of executive and behavioral dysfunction on the course of ALS. Neurology. 2005;65:1774–7.
- Mantovan MC, Baggio L, Dalla Barba G, Smith P, Pegoraro E, Soraru G, et al. Memory deficits and retrieval processes in ALS. Eur J Neurol. 2003;10:221–7.
- Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, et al. Slow saccades in bulbar-onset motor neurone disease. J Neurol. 2010;257:1134–40.
- Witiuk K, Fernandez-Ruiz J, McKee R, Alahyane N, Coe BC, Melanson M, et al. Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. J Neurosci. 2014;34:14260–71.
- Tombaugh TN. Trail making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14.
