Abstract
Background: Radicava® (edaravone), approved for the treatment of amyotrophic lateral sclerosis (ALS) in 2017, may be administered intravenously at clinic sites, infusion centers or at home.
Objective: To gain insights into the utilization of Radicava® at 1 year post-launch.
Methods: Radicava® usage data were collected, and a survey was conducted among 75 physicians. Adverse events (AEs) were identified from a post-marketing safety database from 8 August 2017 through 3 August 2018 (cutoff date).
Results: As of 6 August 2018, 3007 ALS patients were treated with Radicava®. Survey results indicated that 43% of patients received infusions at home, 32% in a clinician’s office, and 26% at a referred site. Infusions were administered mainly via implanted port. The most commonly reported AEs were drug ineffective, death (not specified), therapeutic response unexpected, asthenia, fatigue, gait disturbance, disease progression, muscular weakness, fall, and dyspnea.
Conclusions: The first year of Radicava® availability to ALS patients in the US provided many key learnings that will help shape strategies for improved patient care.
Keywords:
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive and fatal neuromuscular disease, characterized by degeneration of upper and lower motor neurons and is associated with a mortality rate of 50% within 30 months of symptom onset (Citation1,Citation2). There is no cure for ALS; the mainstay of treatment is symptom management and palliative care (Citation3). Riluzole, in use since 1995, has been shown to modestly extend the survival of patients with ALS by 2–3 months (Citation4).
Radicava® (edaravone) was approved by the FDA for the treatment of ALS in May 2017 and became available to US health care providers in August 2017 (Citation5). It is the first new agent able to slow the rate of functional decline (compared with placebo) that has been approved for ALS in the past 22 years (Citation6). Radicava® is administered by infusion at clinic sites and infusion centers, or at home. It is administered once daily in a 60-mg dose over 60 minutes. Infusions are given for 14 consecutive days in the first treatment cycle and for 10 out of the first 14 days of each successive 4-week treatment cycle.
The objective of this study was to gain further insights into the utilization of Radicava® at 1-year post-launch, including real-world data on adverse events (AEs) from postmarketing pharmacovigilance reports. This article also provides insights gained from experience with the use of Radicava® in the authors’ clinical practices during the first year of product availability.
Methods
Radicava® usage
Radicava® use was calculated based on prescription data.
Physician survey
An independent, blinded survey was conducted in April 2018 among 75 US physicians who cared for ALS patients. The survey was conducted by a third party research company and only aggregated survey results were provided to the sponsor (Mitsubishi Tanabe Pharma America, Inc, Jersey City, NJ); the identities of the responding physicians were not provided to the sponsor. In addition, the sponsor was not disclosed to the physicians participating in the survey. Physicians were selected on the basis of medical claims data showing visits with patients diagnosed with ALS, riluzole prescribing, or ordering of electrodiagnostic procedures. Riluzole prescribing data were obtained from dispensers of retail drugs, such as large pharmacy chains. Medical claims and electrodiagnostic procedure data were obtained from commercial and Medicare insurers. Physicians were invited to participate either via email or phone. They were eligible to participate if they had been in practice for at least 2 years, were board certified or board eligible, were caring for at least one ALS patient, and were not employed by any pharmaceutical health care company.
Pharmacovigilance
Cumulative patient cases and AEs were identified from a postmarketing safety database from 8 August 2017 through 3 August 2018 (cutoff date). The safety database includes safety reports received by Mitsubishi Tanabe Pharma from all sources, including spontaneous (voluntary) reports as well as reports from solicited sources (e.g. Mitsubishi Tanabe Pharma-sponsored programs, such as patient support programs). Reports can be submitted by a variety of sources, including physicians, nurses, and consumers. Frequencies of total AEs, serious AEs (SAEs), non-serious AEs, and deaths were tabulated.
Results
Radicava® usage at 1 year after launch
Usage data indicated that 3007 ALS patients had been treated with Radicava® as of 6 August 2018. Of these, an estimated 1006 patients discontinued treatment during that time. In aggregate, based on physician feedback, the reasons for discontinuation included disease progression, influence of the prescribing physician, insurance and financial burden, caregiver burden, clinical trials, and side effects. However, patient-specific information, such as age, disease duration, specific side effects, etc., were not available because the data were derived from de-identified product order information. The average duration of therapy for patients starting treatment in the first year was approximately 199 days, or 6.5 months (range, 3 days to 12 months). During this first year of availability, approximately 936 physicians prescribed Radicava®, with an average of each physician treating three patients with Radicava®.
Physicians participating in the survey
A total of 75 physicians participated in the survey. These physicians were geographically dispersed, representing 29 states in the United States. As shown in , the majority (84%) of the surveyed physicians were neurologists, with 70% identifying as neuromuscular specialists and 52% identifying as ALS specialists. Approximately half of the respondents worked in private practice (group or individual; 53%), and a majority of the remaining respondents worked in academic medical centers (32%). More than half (57%) of the respondents practiced in an urban setting. Of the 75 participating physicians, 40 (53%) were prescribers of Radicava®. The specialties of the physicians who prescribed Radicava® included neurology (83%), physical medicine and rehabilitation (8%), and internal medicine/general practice (5%). Survey respondents indicated that 59% of their patients had inquired about and discussed Radicava® with them.
Table 1 Characteristics of survey respondents.
Use of Radicava® with riluzole
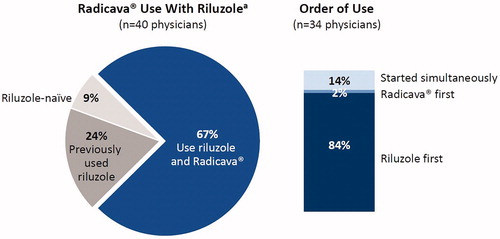
As Radicava® and riluzole are the only FDA-approved medications for ALS, it was of interest to learn how these two agents are being used in clinical practice. Survey respondents indicated that 67% of their Radicava® patients were also receiving riluzole, and the majority began receiving riluzole first ().
Figure 1 The pie chart on the left shows physician survey data from the 40 physicians who administered Radicava®, showing rates of use with riluzole. The graph on the right represents the order of use of Radicava® and riluzole for the 34 physicians who reported using both agents with their patients. aData in the pie chart represent the percentage of patients receiving Radicava® at 9 months after introduction (i.e. as of 6 April 2018).
Administration of Radicava®
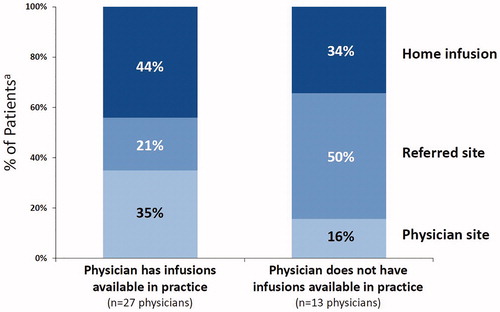
The physician survey revealed that 59% of the patients receiving Radicava® had an implanted port, 21% utilized a peripherally inserted central catheter (PICC), 18% used a peripheral line, and 2% used other methods (). With regard to site of care, 43% of patients received infusions at home, while 32% received them in a health care provider’s office, and 26% went to a referred site (e.g. infusion center). Patients were most likely to use home infusion even if their health care provider offered infusion services in their practice ().
Figure 2 The pie charts show physician survey data from the 40 physicians who administered Radicava®, showing the method of administration (left chart) and the site of administration (right chart). aData in the pie charts represent the percentage of patients receiving Radicava® at 9 months after introduction (i.e. as of 6 April 2018).
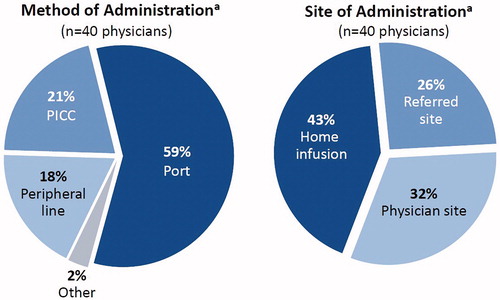
Figure 3 The graph shows physician survey data on site of infusion from the 40 physicians who administered Radicava®, based on whether the physician had infusions available in their practice (27 physicians, left columns) or no infusion facility available in their practice (13 physicians, right columns). aData in the graph represent the percentage of patients receiving Radicava® at 9 months after introduction (i.e. as of 6 April 2018). Note that some physicians who did not have infusions available in their practice may have provided infusions through a partnership with another clinic or through on-site infusion delivery services (i.e. referring to the 16% who utilized the physician site for physicians who did not have infusions available in their practice).
Radicava® safety reports during the first year of treatment
US postmarketing sources reported 817 safety incidents by the cutoff date (3 August 2018). Of the 1660 AEs, 391 were serious (death, life-threatening, hospitalization, disability, other medically significant event) ( and ). The most commonly reported AEs (≥50 reports) were drug ineffective, death (not specified), therapeutic response unexpected, asthenia, fatigue, gait disturbance, disease progression, muscular weakness, fall, and dyspnea (). The most commonly reported SAEs (≥10 reports) were death (not specified), dyspnea, pneumonia, fall, ALS, incorrect dose administration, respiratory failure, and disease progression (). There were 150 cumulative death cases, with 104 cases reported as “death” (without specifying the fatal event). Many of these reports had missing information or were attributed to ALS progression or respiratory causes. Review for serious anaphylactic reaction/hypersensitivity revealed one report (a man who experienced trouble breathing/swallowing after his 6th infusion, which resolved with steroid treatment). Review for serious thromboembolic events revealed six deep vein thrombosis, six pulmonary embolism (), and three thrombosis. Review for site reactions (catheter, injection, infusion) revealed nine serious reports of injection site infection (). There were two serious hepatic cases (one hepatic enzyme increased, one liver disorder) and two serious renal cases (two renal failure) reported.
Table 2 Adverse events reported.
Table 3 Serious adverse events reported.
Discussion
As Radicava® is the only treatment option approved in the US to slow the decline in physical function in ALS, there is considerable interest in how this drug is being utilized in clinical practice. For example, the most recent Motor Neurone Disease Association symposium held in Glasgow, Scotland in December 2018, included approximately 20 presentations (posters and oral presentations) related to the clinical use of edaravone (https://www.mndassociation.org/symposium). Consideration must be taken in assessing these presentations because not all of the studies utilized edaravone manufactured by Mitsubishi Tanabe Pharma (i.e. Radicava® and Radicut®).
Regarding the use of Radicava® in the US, this physician survey indicated that, although Radicava® was being administered mostly by neurologists, various other types of clinicians were also administering it, including those specializing in physical medicine and rehabilitation and those in internal medicine or general practice.
The majority of patients receiving Radicava® were also receiving riluzole, consistent with the belief that these agents have complementary mechanisms of action. This is also consistent with the experience of the clinical authors of this study. Often, both drugs are prescribed at the time of diagnosis. As riluzole is readily available by prescription, most patients begin receiving riluzole almost immediately and then begin receiving Radicava® when approved by their insurance provider. The American Academy of Neurology Practice Guidelines recommend that riluzole be offered to all patients with ALS based on Class 1 evidence (Citation3). Many patients, however, elect not to take riluzole due to lack of perceived benefit, cost, or side effects. Patients should be advised of all treatment options and through shared decision making, decide upon the treatments they wish to try. As with any therapeutic regimen with more than one drug, it is important to allow sufficient time to assess side effects of one medication before starting another medication.
The survey indicated that 43% of patients received Radicava® administration at home, with the remaining receiving administration at a clinician’s office or a referred site. More than half (59%) of the patients receiving Radicava® had an implanted port. In the authors’ experience, most patients receive their first infusion of Radicava® at a clinical site so that they can be adequately monitored for adverse reactions. As indicated by the pharmacovigilance data, only one serious case of anaphylactic reaction/hypersensitivity was reported through 3 August 2018, and this reaction resolved with steroid treatment. After the first infusion cycle, most patients are able to receive subsequent infusions of Radicava® at home through an implanted port.
In the pharmacovigilance reports, the most commonly reported AEs were drug ineffective, death (not specified), therapeutic response unexpected, asthenia, fatigue, gait disturbance, disease progression, muscular weakness, fall, and dyspnea. The reported AEs are not qualitatively different from those reported in other ALS trials or from ALS disease progression. In addition, there is an inherent challenge in interpreting AEs commonly associated disease progression, as ALS is not a linear disease (Citation7–9). While edaravone has been shown to slow physical functional decline compared with placebo, it is not a cure; therefore, progression of disease is expected.
Further studies are being planned in patients treated with Radicava® to determine the medication’s impact on available biomarkers and to evaluate potential changes in the dosing regimen. It is important to carefully monitor each patient’s disease progression and functional status and continue a dialog with the patient and their caregivers in order to provide appropriate care for patients with ALS.
Limitations
The physician survey included a limited sampling of physicians (75); although, they were selected according to their experience with managing patients with ALS, and they were located at a variety of sites across the United States. The pharmacovigilance data, based on spontaneous reports of AEs, by definition, should be interpreted with this in mind.
Conclusions
The first year of Radicava® availability to ALS patients in the US provided many key learnings regarding the use of this drug. In clinical practice, patients can receive infusions at home. These learnings continue to shape strategies for streamlining the process of delivering improved care in ALS.
Declaration of interest
CJ serves as a consultant for Alexion, Avexis, Cytokinetics, ITF Pharmaceuticals, MTPA, and Strongbridge Pharmaceuticals, receives research support from Amylyx, Cytokinetics, Muscular Dystrophy Association, and the National Institute of Neurological Disorders and Stroke, and serves on the Data Safety Monitoring Board for Anelixis, Brainstorm, and Mallinckrodt. THP reports grants and personal fees from MTPA, outside the submitted work. PK has no declarations of interest to report. TB, GM, WA, and MM are employees of MTPA. LB is an employee of Mitsubishi Tanabe Pharma Development America, Inc.
Acknowledgments
The study was conducted by MTPA. Radicava and MTPA are registered trademarks of Mitsubishi Tanabe Pharma Corporation, Inc. Medical writing assistance was provided by p-value communications.
Additional information
Funding
References
- Brooks BR, Jorgenson JA, Newhouse BJ, Shefner JM, Agnese W. Edaravone in the treatment of amyotrophic lateral sclerosis: efficacy and access to therapy – a roundtable discussion. Am J Manag Care. 2018;24:S175–86.
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–72.
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1218–26.
- Rilutek® (riluzole) [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2012.
- Radicava® (edaravone injection) [package insert]. Jersey City, NJ: Mitsubishi Tanabe Pharma Corporation; 2018.
- Beydoun SR, Rosenfeld J. Edaravone in amyotrophic lateral sclerosis—lessons from the clinical development program and the importance of a strategic clinical trial design. US Neurol. 2018;14:47–53.
- Fournier CN, Schoenfeld D, Berry JD, Cudkowicz ME, Chan J, Quinn C, et al. An open label study of a novel immunosuppression intervention for the treatment of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:242–9.
- Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:414–25.
- Thakore NJ, Lapin BR, Kinzy TG, Pioro EP. Deconstructing progression of amyotrophic lateral sclerosis in stages: a Markov modeling approach. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:483–94.
