Abstract
Objective
To explore novel, real-world biotelemetry disease progression markers in patients with amyotrophic lateral sclerosis (ALS) and to compare with clinical gold-standard measures. Methods: This was an exploratory, non-controlled, non-drug 2-phase study comprising a variable length Pilot Phase (n = 5) and a 48-week Core study Phase (n = 25; NCT02447952). Patients with mild or moderate ALS wore biotelemetry sensors for ∼3 days/month at home, measuring physical activity, heart rate variability (HRV), and speech over 48 weeks. These measures were assessed longitudinally in relation to ALS Functional Rating Scale-Revised (ALSFRS-R) score and forced vital capacity (FVC); assessed by telephone [monthly] and clinic visits [every 12 weeks]). Results: Pilot Phase data supported progression into the Core Phase, where a decline in physical activity from baseline followed ALS progression as measured by ALSFRS-R and FVC. Four endpoints showed moderate or strong between-patient correlations with ALSFRS-R total and gross motor domain scores (defined as a correlation coefficient of ≥0.5 or >0.7, respectively): average daytime active; percentage of daytime active; total daytime activity score; total 24-hour activity score. Moderate correlations were observed between speech endpoints and ALSFRS-R bulbar domain scores; HRV data quality was insufficient for reliable assessment. The sensor was generally well tolerated; 6/25 patients reported mostly mild or moderate intensity skin and subcutaneous tissue disorder adverse events. Conclusions: Biotelemetry measures of physical activity in this Pilot Study tracked ALS progression over time, highlighting their potential as endpoints for future clinical trials. A larger, formally powered study is required to further support activity endpoints as novel disease progression markers.
Keywords:
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting motor neurons, characterized by progressive muscle weakness leading to impaired speech, swallowing, mobility, and breathing (Citation1). Development of novel therapies depends on sensitive and reliable measures of disease progression; however, current clinical testing measures have limitations. The ALS Functional Rating Scale-Revised (ALSFRS-R), a gold-standard measure of functional decline routinely used to monitor disease progression and evaluate treatment effects in clinical trials and clinical practice (Citation2–4), provides a measure of patients’ subjective perception of function. Spirometry, also used for routine clinical monitoring, can be technically difficult, especially in patients with bulbar symptoms, and requires repeated in-clinic assessments, increasing patient burden. For this reason, there has been an increasing interest in investigating new technologies for remote and objective assessment of clinical parameters in ALS that may be useful as outcome measures in clinical trials as well as in practice. Objective biotelemetric parameters have potential as novel “digital” biomarkers to quantify changes in function and disease progression with greater sensitivity than current clinical methods, thus ultimately enabling smaller and shorter trials. In addition, this approach could be helpful in assessing the impact of the disease and treatment on clinical function and activities of daily living in a real-life setting, while reducing the testing burden.
This pilot study, exploring the use of novel biotelemetry measures of clinical function in ALS in comparison with the gold-standard assessments, aimed to identify objective measures of ALS progression. This approach has also been recognized in the recent industry guidance on ALS drug development from the US Food and Drug Administration, which encourages the investigation of technologies for remote patient monitoring as well as exploring the use of digital biomarkers as clinical trial endpoints (Citation5).
Materials and methods
Study design
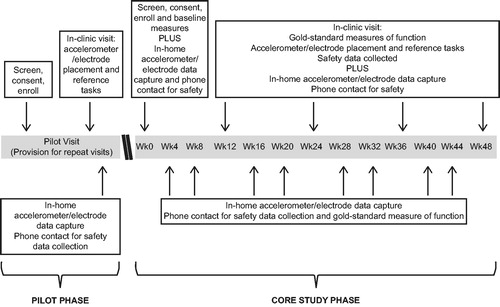
This was an exploratory, non-controlled, non-drug study (GSK study 201283; NCT02447952). The study consisted of a variable length Pilot Phase and a 48-week Core Study Phase (). A total of 5 patients were recruited for the Pilot Study Phase; these patients attended 2 clinical site visits and performed one remote home-monitoring period. This Study Phase focused on the assessment of feasibility of the approach including early development and evaluation of data analysis algorithms, refinement of equipment and data transmission processes, and assessment of patient tolerability and wearable sensor acceptance before starting the Core Study Phase (Citation6). The Core Study Phase investigated the biotelemetry measures of movement or physical activity, heart rate variability (HRV) and speech longitudinally, and explored their relationship with the clinical gold-standard measures of ALS progression. Patients attended in-clinic visits every 12 weeks to perform gold-standard measures of function and wore the sensor in their daily life continuously for ∼3 days every month (except ∼2 hours per day for device re-charge). Patients were contacted by telephone at the end of every home-monitoring period for administration of ALSFRS-R and to report adverse events (AEs).
The monitoring system utilized comprised three main components: 1) The commercially available Mega Faros 180 accelerometer (3 axes, 50 Hz) and 2-lead ECG sensor (Mega Electronics Ltd., Finland) which was attached to patient's torso during the remote monitoring periods, 2) A “LifeInsight Hub (v.2.0.6.)” developed by McLaren Applied Technologies, which received data from the sensor via a secure Bluetooth and automatically uploaded the data in near-real-time to secure cloud servers via a secure connection on a mobile phone network and 3) a bespoke digital speech capture system comprising a high-fidelity microphone connected to a computer with customized software.
Additional details of the devices and data transmission systems are provided in Supplementary Materials and Supplementary Figure 1.
Patients
This study included patients diagnosed with ALS by a neurologist with ALS expertise within 18 months of symptom onset, who were ambulant and therefore were expected to have a relatively high level of clinical function at baseline. Eligible patients were aged 18–80 years, capable of giving informed consent and capable of and willing to comply with the protocol. Exclusion criteria and withdrawal criteria are provided in Supplementary Materials.
Study endpoints
Exploratory physical activity endpoints aimed to evaluate patients’ ability to perform activities of daily living and included: time spent active, time spent sedentary, total activity score (reflecting amount and intensity of movement), number and duration of active periods as well as nighttime-specific rest measures, such as number of nighttime movements per hour. The HRV endpoints aimed to assess cardiac autonomic control and included time-domain (e.g. Root Mean Square of the Successive Differences, RMSSD) and frequency-domain (e.g. low-frequency/high-frequency [LF/HF] component ratio) metrics. Digital speech endpoints, such as jitter, shimmer, or speaking rate, aimed to evaluate speech.
Changes over time for all exploratory endpoints, relationships between the exploratory endpoints and ALSFRS-R (total and domain scores), and between the speech endpoints and forced vital capacity (FVC) were examined. Feasibility of the novel biotelemetry platform is presented in Garcia-Gancedo et al. (Citation6). Safety evaluation consisted on reporting AEs and serious AEs (SAEs) deemed related to the study equipment, devices, or procedures only by investigator.
Further details of measurements are provided in Supplementary Materials.
Statistical analyses
A total of 25 patients were enrolled to allow 20 evaluable patients (anticipating 20% drop-out rate).
FVC, speech, HRV, and physical activity data were analyzed as absolute values, change from baseline, and relative and monthly rates of change (Supplementary Materials). Between-patient and within-patient correlations (see Supplementary Materials) were analyzed by fitting a multiple linear regression model between the exploratory endpoints and ALSFRS-R score, and between speech endpoints and FVC (Citation7). Twenty patients were anticipated to provide an 80% chance of detecting a within-patient correlation of ≥0.6, if the true correlation was 0.7.
Correlation coefficients between endpoints were categorized as weak (<0.5), moderate (≥0.5 to 0.7), or strong (>0.7). Data were analyzed by length of recording periods and filtered to avoid inclusion of data from short recording periods that would not be representative of a patient’s day. For the actigraphy sensor, “quality data” was defined as containing a minimum wear time of 70% of the day, based on previous studies (Citation8,Citation9), and 60% at night. Because fewer data were recorded for HRV than expected, the required quality data percentage was lowered to >40% data per 24-hour period.
To assess the utility of the exploratory endpoints for future studies, sample size calculations as part of exploratory statistical power analyses for a mock clinical trial were performed using a two-sided, two sample t-test procedure with a type I error rate of 5% and a power target of 80%, assuming a monotonic decline in outcome measure and a study duration of 48 weeks.
Standard protocol approvals, registrations, and patient consent
The study protocol, any amendments, and informed consent forms were reviewed and approved by the National Research Ethics Service Committee South Central – Berkshire (Ref No. 15/SC/0080), in accordance with the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice and applicable country-specific requirements. Each patient gave written informed consent prior to any study-specific procedures.
Results
Pilot phase
During the Pilot Study Phase, the amount of data received by the secure server gave sufficient confidence that the system was working correctly, although HRV data quality was lower than anticipated. Most patients found the device comfortable to wear; one patient experienced moderate skin irritation. None reported that wearing the sensor had an impact on daily activity performance. Further details are included in Garcia-Gancedo et al. (Citation6). These data were sufficiently promising to warrant progression to the Core Study Phase with minimal modifications to the monitoring platform (no protocol amendments were necessary).
Core study phase
Patient demographics
Twenty-five patients (aged 32–71 years; 84% male) were enrolled in the Core Study Phase, including the 5 patients from the Pilot Study Phase (). The mean (standard deviation) baseline ALSFRS-R score was 41.6 (4.98). Twenty-three patients (92%) had limb-onset ALS and two patients (8%) had bulbar-onset ALS.
Table 1 Patient demographics and baseline characteristics.
Eighteen of the 25 patients completed the study. Of the 7 patients that did not complete, 4 withdrew consent (3 of whom had become too unwell to continue due to ALS progression), 2 withdrew due to AEs, and 1 withdrew at the investigator’s discretion (also had become too unwell to continue due to ALS progression). The mean (standard error [SE]) monthly rate of change for ALSFRS-R total score was −0.9 points/month (0.23), decreasing from 41.6 (1.00) at baseline to 35.2 (1.30) at Week 48. ALSFRS-R total score for those who had an Early Withdrawal Visit (n = 5) was 28.0 (6.47). FVC demonstrated a mean (SE) monthly rate of change of −0.06 L/month (0.02), decreasing from 3.93 (0.30) at baseline to 3.33 (0.38) by Week 48. Data collection is described in Supplementary Materials.
Technology feasibility
The biotelemetry platform utilized was successful in measuring physical activity of patients: data propagated through the system and were received as expected. However patient observance of protocol requirements was lower than anticipated: many patients did not wear the sensor for the whole duration of the monitoring periods, and this resulted in less data than planned for. The amount and quality of the speech data captured were as intended, and there were no significant issues with the methods, equipment, or the data analysis. However, HRV data quality appeared to be affected by a poor connection between the sensor electrode patch and the chest, yielding insufficient HRV data for robust analyses.
Physical activity
All the activity endpoints investigated changed from baseline over the course of the study to indicate a decline in physical activity over time (). This decline was consistent with the deterioration in ALS disease progression as measured by ALSFRS-R.
Table 2 Actigraphy endpoints.
Four physical activity endpoints of interest were identified, for which quality data showed trends over time that were considered clinically relevant, and for which the strongest correlations with the ALSFRS-R total score (between-patient correlation coefficient, rT, of ≥0.5) and ALSFRS-R gross motor domain score (between-patient correlation coefficient, rGM, of ≥0.7) were observed: average time spent active in the daytime (rT=0.500, rGM=0.726), percentage of time spent active in the daytime (rT=0.514, rGM=0.716), total daytime activity score (rT=0.529, rGM=0.732) and total 24-hour activity score (rT=0.499, rGM=0.707; and ; ). Similar findings were observed when analyzing the matched completers population (n = 13), although only weak within-patient correlations were observed. For these four endpoints of interest, the relative rate of change measured by the actigraphy device was similar to or greater than that measured by ALSFRS-R total and gross motor domain scores; however, the actigraphy data had the greatest variability (). It is noteworthy that “activity” was algorithmically defined as any period in which the participants performed any activity that had equal to or greater intensity than “walking at average pace”. Modifying this definition to a higher or lower threshold returns lower or higher absolute values of these endpoints, but changes over time are anticipated to follow a similar trend.
Figure 2 Correlation of the four physical activity endpoints of interest to the ALSFRS-R total score*. The between-patient correlations (r) and n numbers (n [number of patients with available data at the time point] and N [number of patients in the study at the time point]) are indicated on each graph. ALSFRS-R; amyotrophic lateral sclerosis functional rating scale – revised; BL: baseline; CL: clinic; SE: standard error; TC: telephone contact. *Quality data population, which included patients with data meeting the quality data criteria (see Methods section).
![Figure 2 Correlation of the four physical activity endpoints of interest to the ALSFRS-R total score*. The between-patient correlations (r) and n numbers (n [number of patients with available data at the time point] and N [number of patients in the study at the time point]) are indicated on each graph. ALSFRS-R; amyotrophic lateral sclerosis functional rating scale – revised; BL: baseline; CL: clinic; SE: standard error; TC: telephone contact. *Quality data population, which included patients with data meeting the quality data criteria (see Methods section).](/cms/asset/ca28e714-8625-44ce-9a14-911c140e0e7e/iafd_a_1773501_f0002_c.jpg)
Figure 3 Correlation of the four physical activity endpoints of interest to the ALSFRS-R gross motor domain score*. The between-patient correlations (r) and n numbers (n [number of patients with available data at the time point] and N [number of patients in the study at the time point]) are indicated on each graph. ALSFRS-R: amyotrophic lateral sclerosis functional rating scale – revised; BL: baseline; CL: clinic; SE: standard error; TC: telephone contact. *Quality data population, which included patients with data meeting the quality data criteria (see Methods section).
![Figure 3 Correlation of the four physical activity endpoints of interest to the ALSFRS-R gross motor domain score*. The between-patient correlations (r) and n numbers (n [number of patients with available data at the time point] and N [number of patients in the study at the time point]) are indicated on each graph. ALSFRS-R: amyotrophic lateral sclerosis functional rating scale – revised; BL: baseline; CL: clinic; SE: standard error; TC: telephone contact. *Quality data population, which included patients with data meeting the quality data criteria (see Methods section).](/cms/asset/473d6b9f-4aa3-45f9-a208-02d99956994d/iafd_a_1773501_f0003_c.jpg)
Table 3 Correlation coefficients between Actigraphy endpoints of interest and ALSFRS-R.
Table 4. Relative rate of change of activity endpoints versus ALSFRS-R.
Exploratory statistical power analyses for a mock clinical trial, comparing an active treatment with placebo, were performed using the observed monthly rates of decline versus those calculated for the ALSFRS-R total score, assuming a 30% reduction in rate of decline. For the ALSFRS-R total score, a sample size of 290 patients per arm was estimated to be required, while 500–700 patients per arm was estimated for the physical activity endpoints when assessed according to the specific parameters of this study design and sensor technology ().
Table 5 Sample size estimates for use of activity endpoints in clinical trials.
The duration of activity periods was short and the mean number of activity periods per hour was low at baseline, with both these endpoints decreasing slightly over the course of the study (). No patient recorded daytime active periods of >30 minutes, and most frequently recorded daytime active periods lasted >1 to ≤2 or >2 to ≤5 minutes. Average daytime active time was 43.9 minutes at baseline; however, average duration of active periods lasting >1 minute was 2.5 minutes and the number of active periods (>1 minute) was 0.29 per hour, indicating patients were mostly active for <1 minute at a time.
There was a corresponding small increase in the percentage of time spent sedentary in the daytime over the course of the study (baseline 94.5%, Week 48 96.4%), with moderate-to-strong between-patient correlations versus the ALSFRS-R total score and the gross motor domain score; these findings were not observed for duration of time spent sedentary. The number of nighttime movement episodes decreased over the course of the study; however, the data were more variable at each consecutive time point, potentially due to smaller sample size.
Heart rate variability
During the Core Study Phase, the quantity of HRV data captured was too low to draw any definitive conclusions. A slight decrease in the RMSSD HRV mean and variance data was observed over time, but this finding should be treated with caution given the small sample size (4 patients with quality data at Week 48). There was little change in the absolute mean LF/HF values over the course of the study, though data were limited, and levels of variability were high across all measurements taken.
Speech
Four speech endpoints had between-patient correlation coefficients ≥0.5 with the bulbar (average phoneme rate and average speaking rate) and respiratory domain scores (jitter and shimmer) of the ALSFRS-R. However, examination of absolute values over the course of the study showed no definitive pattern of change, with high variability (Supplementary Figure 2). The correlation between speech data and FVC only showed between-patient correlation coefficients ≥0.5 in average speaking rate.
Safety
The biotelemetry equipment was generally well tolerated. In the Pilot Phase, one moderate skin and subcutaneous tissue disorder AE was reported by 1 patient. In the Core Phase, 21 AEs were reported by 6 patients; all skin and subcutaneous tissue disorders (). Events were mild or moderate in 5/6 patients and resolved by study end. One patient experienced 2 severe AEs of pruritic rash; the first lasted for 4 days and the second for 5 days; both resolved and the patient completed the study. There were no deaths or SAEs; 2/25 patients withdrew from the study due to AEs (dermatitis).
Table 6 Adverse events.
Further safety information has been included in Supplementary Materials.
Discussion
The novel biotelemetry platform used in this study was sensitive to change in the daily physical activity of patients with ALS, providing insights into physical activity in a real-life setting. Mean ALSFRS-R total score at baseline suggested study participants had mild-to-moderate functional impairment. The actigraphy device detected a gradual decline in physical activity across all activity endpoints over the course of the study, consistent with ALS disease progression assessed by ALSFRS-R. In particular, a moderate correlation was found between ALSFRS-R total score and the four activity endpoints (average time spent active in the daytime; percentage of time spent active in the daytime; daytime total activity score; 24-hour total activity score), and a strong correlation was found between ALSFRS-R gross motor domain score and these four endpoints, highlighting them as key endpoints of interest for further investigation. These endpoints also showed greater median relative rates of decline than those for the ALSFRS-R scores, suggesting actigraphy may be a more sensitive measure of disease progression.
Although the quantity and quality of speech data captured by the biotelemetry platform were as intended, no consistent changes over time were noted for any of the speech endpoints. This contrasts with previous publications reporting speech decline with ALS, measured by endpoints including speech intelligibility, speaking rates, and voice measures (e.g. jitter, shimmer, and volume) (Citation10,Citation11). However, 92% of patients in the current study had limb-onset ALS, whereby speech deterioration may be variable and arise later in the disease course, compared with bulbar-onset ALS (Citation12), highlighted by the limited progression of the bulbar domain score (and in particular the Speech item) of ALSFRS-R during this study.
HRV measures cardiac autonomic modulation, including both parasympathetic and sympathetic influences (Citation13). Patients with ALS have impaired cardiac autonomic control, with marked parasympathetic dysfunction and sympathetic predominance (Citation14). Although our results seem to suggest a slight decrease in the HRV RMSSD parameter over time in patients with ALS (which may indicate an increase of the relative predominance of sympathetic activity of the autonomic nervous system with disease progression), this effect was very small, and it is difficult to draw meaningful conclusions particularly considering the limited HRV data available.
While this study had encouraging results with physical activity endpoints, data collection was subject to several limitations (quality and quantity). Since the study start, a number of advances were made in the field of wearable technology. Using technologies that are not intrusive, awkward or require frequent interaction with patients has been shown to enhance data quantity: some wrist-worn devices have shown excellent patient acceptance in recent studies; however, they are currently unable to monitor HRV continuously; and from a biomechanical perspective, the wrist is a less-than-ideal sensor location for accurate activity classification (Citation15). Chest-worn patch-like devices remain the best option to accurately and simultaneously quantify physical activity and continuous HRV. Recent technological advances have enabled chest-worn devices to be available with a more reliable skin attachment and not needing to be recharged daily, thereby significantly improving data quality. Moreover, future studies may employ mobile phones as a mean to i) collect digital speech data at home and more frequently and ii) serve as a “hub” to transmit sensor data to the cloud in near-real-time (replacing the “LifeInsight Hub”), which would enable a fully integrated, remote biotelemetry monitoring system. Unfortunately, these technologies were not sufficiently mature at the time this study was designed.
Overall, physical activity endpoint results were associated with greater variability than ALSFRS-R scores and generally became more variable toward study end, possibly as a consequence of reduced patient numbers as the study progressed. Slight increases in physical activity and ALSFRS-R total scores were observed at Week 36, seemingly due to patients with greater disease progression and hence poorer activity levels being more likely to drop out at this stage; indeed, the ALSFRS-R score for patients who withdrew early from the study was lower than that for study completers.
As a consequence of the data variability, sample size calculations suggest that a larger number of patients would be required to design a clinical study powered by these activity endpoints (using an identical study design and sensor) compared with ALSFRS-R; thus, further methodological refinements are needed before these endpoints can be recommended as novel markers of disease progression. It is important to note that the study ALSFRS-R data used for the sample size calculation also showed considerable variability, resulting in a greater required sample size compared with earlier clinical studies (Citation16). Digital endpoint variability can arise from factors such as study design including a small sample size, duration of the monitoring periods (3 days may not be representative of patients typical behavior, longer monitoring periods may be needed), patient compliance with the technology (many patients wore the device for less than 3 days, or less than the 22h per day that was recommended and as a consequence, the data collected per monitoring period may be even less representative of patients typical behavior), as well as the intrinsic variability of the endpoint. For this reason, the sample size estimates presented here need to be considered within the context of this particular study design and technology. The use of less obtrusive and easier-to-use technologies will enable data collection over longer monitoring periods without additional patient burden, which will result in lower data variability and subsequent lower sample size estimates for the same endpoints. Recent work by van Ejik et al. showed lower activity-based endpoint variability than ALSFRS-R using a hip-worn activity device for monitoring periods of 7 days, resulting in lower sample size requirements compared with ALSFRS-R for a follow-up time of >9 months. Of note, estimated sample sizes for ALSFRS-R per group were greater (around 500 in the publication compared to 290 in the present study), probably due to the smaller rate of decline (Citation17).
This exploratory study indicates that biotelemetry could help overcome some limitations of the current gold-standard measures of ALS. Comparatively, biotelemetry captures direct, objective real-life physical measurements, which are data-rich thus improving precision, and do not rely on subjective factors such as patient recall (such as ALSFRS-R) or impressions by the patient, carer or healthcare provider. Platforms incorporating multiple clinical monitoring technologies present the best opportunity to develop multiple digital biomarkers – and composite digital biomarkers combining measures across different domains that may be a more sensitive marker of disease progression than individual measures, and ultimately than the current clinical “gold standard”.
In conclusion, this study applied a novel technology to monitor patients with ALS, providing unique real-world insights into the physical activity of patients in their daily environment. Four physical activity endpoints showed potential for use as clinical measures of ALS disease progression, using direct, objective, and real-life assessment of physical function. Although larger-scale validation is required, these exploratory findings highlight a promising potential of the biotelemetry platform as an efficient clinical evaluation tool of disease progression in patients with ALS, with pertinent application in maximizing the efficiency of clinical studies of emerging ALS treatments.
Author contributions
Study conception or design: MK, AL, JP, CES, AAC, MRT, KT. Acquisition of data: AL, LGG, JP, RH, CES, AAC, RM, MRT, KT. Data analysis or interpretation: MK, AL, LGG, JP, RH, TC, CES, AAC, MRT, KT.
Supplementary_Materials_ALS-FTD_submission_review2.docx
Download MS Word (687.1 KB)Acknowledgements
The authors sincerely thank the patients who participated and their families; the authors are extremely grateful for their efforts in the study. The authors thank all the investigators and members of the site teams, as well as the team at McLaren Applied Technologies who developed the technology for this study. The authors thank Theodora Adamou for her involvement in the data analysis, Jane Temple for her input on the study design, Jo Barnard and Alison Peacock for their clinical operational input, and Michelle Crouthamel for her support in managing the project (affiliation for all at the time of the study: GSK). Medical writing support in the form of developing drafts based on author input, editorial assistance, and submission of the final manuscript was provided by Leigh O’Connor, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
Declaration of interest
LGG is an employee of GSK and holds stocks/shares. MK and AL were employees of GSK at the time of the study, data analysis and data interpretation, and still hold stocks/shares. CES has previously consulted for GSK (>5 years ago), has received research grants from Vertex and Chronos Therapeutics in the past, and has an active grant with Eli Lily. AAC reports consultancies for GSK, Cytokinetics, Biogen Idec, Treeway Inc., Chronos Therapeutics Ltd, OrionPharma, and Mitsubishi-Tanabe Pharma, and was Chief Investigator for commercial clinical trials run by OrionPharma and Cytokinetics. JP, RH, TC, RM, MRT, and KT report no disclosures.
Data availability statement
Anonymised individual patient data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Additional information
Funding
References
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–72.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21.
- Paganoni S, Cudkowicz M, Berry JD. Outcome measures in amyotrophic lateral sclerosis clinical trials. Clin Investig (Lond). 2014;4:605–18.
- Simon NG, Turner MR, Vucic S, Al-Chalabi A, Shefner J, Lomen-Hoerth C, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76:643–57.
- Food and Drug Administration. Amyotrophic lateral sclerosis: developing drugs for treatment. Guidance for industry [online]. 2019 [accessed 2019 Nov 22]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/amyotrophic-lateral-sclerosis-developing-drugs-treatment-guidance-industry
- Garcia-Gancedo L, Kelly ML, Lavrov A, Parr J, Hart R, Marsden R, et al. Objectively monitoring amyotrophic lateral sclerosis patient symptoms during clinical trials with sensors: observational study. JMIR Mhealth Uhealth. 2019;7:e13433.
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1 – correlation within subjects. BMJ. 1995;310:446.
- Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Prev Chronic Dis. 2012;9:E113.
- Tudor-Locke C, Johnson WD, Katzmarzyk PT. U.S. population profile of time-stamped accelerometer outputs: impact of wear time. J Phys Act Health. 2011;8:693–698.
- Yunusova Y, Graham NL, Shellikeri S, Phuong K, Kulkarni M, Rochon E, et al. Profiling speech and pausing in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Plos One. 2016;11:e0147573.
- Rong P, Yunusova Y, Wang J, Green JR. Predicting early bulbar decline in amyotrophic lateral sclerosis: a speech subsystem approach. Behav Neurol. 2015;2015:183027.
- Makkonen T, Ruottinen H, Puhto R, Helminen M, Palmio J. Speech deterioration in amyotrophic lateral sclerosis (ALS) after manifestation of bulbar symptoms. Int J Lang Commun Disord. 2018;53:385–392.
- Ferreira MJ, Zanesco A. Heart rate variability as important approach for assessment autonomic modulation. Motriz Rev Educ Fis. 2016;22:3–8.
- Pavlovic S, Stevic Z, Milovanovic B, Milicic B, Rakocevic-Stojanovic V, Lavrnic D, et al. Impairment of cardiac autonomic control in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11:272–276.
- Andreu-Perez J, Garcia-Gancedo L, McKinnell J, Van der Drift A, Powell A, Hamy V, et. al. Developing fine-grained actigraphies for rheumatoid arthritis patients from a single accelerometer using machine learning. Sensors 2017;17:2113.
- Meininger V, Genge A, van den Berg LH, Robberecht W, Ludolph A, Chio A, et al.; NOG112264 Study Group. Safety and efficacy of ozanezumab in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:208–216.
- van Eijk RPA, Bakers JNE, Bunte TM, de Fockert AJ, Eijkemans MJC, van den Berg LH, et al. Accelerometry for remote monitoring of physical activity in amyotrophic lateral sclerosis: a longitudinal cohort study. J Neurol. 2019;266:2387–2395.