Abstract
In the planning and design of the Radicava/Edaravone Findings in Biomarkers From Amyotrophic Lateral Sclerosis (REFINE-ALS) study, we sought to elicit feedback from patients with ALS and their caregivers to ensure that patient-centric issues would be addressed. Ten ALS Clinical Research Learning Institute (ALS-CRLI) Research Ambassadors participated in 2 meetings. They provided perspectives on patients’ interest in the study, the schedule of study visits, and data sharing. The findings were used to help revise the study design, as appropriate. Key concerns identified were (1) the frequency of sample collections, (2) participant travel burden, (3) enrollment criteria, and (4) data reporting and sharing with participants. Several of the identified issues were promptly addressed. The number of visits was reduced, travel optimized, entry criteria clarified, and plans for sharing participants’ data with them were codified. The feedback from the Ambassadors was substantive and resulted in constructive patient-centric changes to the study protocol.
Introduction
REFINE-ALS (Radicava/Edaravone Findings in Biomarkers From Amyotrophic Lateral Sclerosis) is a prospective, observational, longitudinal study conducted in a broad, real-world population of patients with amyotrophic lateral sclerosis (ALS) treated with edaravone in the United States. The study aims to identify biomarkers that may serve as quantifiable biological, nonclinical measures of the pharmacodynamic effect of edaravone and to collect real-world evidence on the use of edaravone in ALS patients (Citation1).
The planning of REFINE-ALS has been guided by a steering committee consisting of practicing ALS physicians and researchers, which has helped develop, refine, and validate the selection and prioritization of biomarkers and the clinical assessments for the study. In addition, to ensure an appropriate patient-centric focus to the study, we enlisted the assistance of individuals with ALS and their caregivers in its design, a collaborative approach that has demonstrated valuable impacts to ALS clinical research (Citation2–6).
In ALS, patients and their caregivers can train to participate in research study design by participating in voluntary training programs, such as those conducted by the ALS Clinical Research Learning Institute (ALS-CRLI; North America) and the Sheffield Motor Neurone Disorders Research Advisory Group (SMNDRAG; Europe) (Citation7,Citation8). The ALS-CRLI training program, modeled after similar programs in cancer and Parkinson’s disease, is designed to educate and prepare patients and caregivers to participate in the clinical research process as well as awareness campaigns (Citation7). More than 300 program graduates, designated as Research Ambassadors, have been trained over the past decade, and many have participated in lobbying efforts, patient advocacy groups (e.g., National ALS Registry, Department of Defense ALS Research Program), and successful fundraising initiatives that have increased ALS research productivity (Citation7,Citation9,Citation10). Their involvements in ALS clinical research have led to more patient-centric study designs with increased enrollment and retention rates (Citation2,Citation7,Citation11), a benefit that is increasingly recognized by recent ALS guidelines (Citation3).
Therefore, we sought the collaboration of the ALS-CRLI Research Ambassadors to capture and incorporate patient and caregiver perspectives into the study design for REFINE-ALS. This report summarizes the outcome of these meetings and the changes that we made.
Methods
All ALS-CRLI Research Ambassadors were notified of the REFINE-ALS study and were asked whether they would be interested to be part of the planning committee for the study. Those who responded with interest were invited to 2 advisory board meetings conducted during the planning stages of the REFINE-ALS study. At both meetings, Research Ambassadors were provided with detailed information about the study, including the planned study design, biomarkers, and clinical assessments, and were then asked to provide feedback on various aspects of the study (). Important, actionable suggestions were identified, based on the consensus of the group (i.e., general agreement and/or lack of objections from the majority of the participants).
Table 1 Summary of information exchange with the ALS Research Ambassadors on the REFINE-ALS study design.
Results
Altogether, 10 Ambassadors (8 ALS patients, 1 pre-symptomatic ALS gene carrier, 1 caregiver) responded with interest in providing feedback on the REFINE-ALS study design. Response from the Ambassadors were largely uniform in terms of expressing a high level of interest in the study among the ALS patient community and in the goal of the study to elucidate the biological mechanisms of edaravone’s efficacy.
There was consensus among the Research Ambassadors on several aspects of the study. First, frequent study visits and the need to make related travel arrangements (i.e., for patients living far from study centers) were considered a significant burden on patients and caregivers. Second, the Ambassadors wanted REFINE-ALS participants to have the option to receive the results of standard clinical assessments, such as those for the ALS Functional Rating Scale Revised (ALSFRS-R) or measures of vital capacity, preferably in real time or through aggregated reporting at the end of the study. However, they acknowledged that real-time reporting could have either a positive or a negative impact, depending on the results. Sharing of experimental test results (e.g., epigenetic testing) was also advocated, although without broad agreement. For patients who opted to receive genetic testing results (i.e., genomic analysis to identify genes associated with high risk for ALS), they recommended that appropriate genetic counseling be provided.
Based on feedback from the Ambassadors, several substantive changes were made to the study design (). A summary of the changes made to the study protocol based on the feedback is shown in .
Figure 1 Changes to the original study protocol based on feedback from advisory board meetings with the Research Ambassadors.
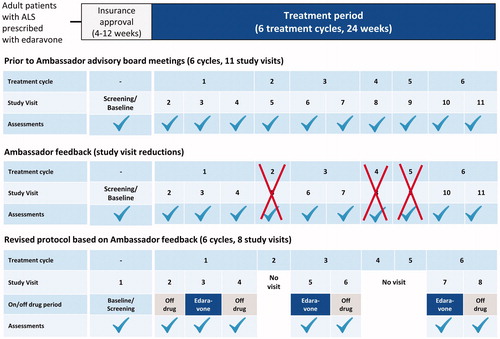
Table 2 Summary of actionable key feedback from the Research Ambassadors and changes implemented to the REFINE-ALS study design as a result.
Discussion and conclusions
REFINE-ALS has proactively involved ALS Research Ambassadors in its planning and design. Overall, there was a wealth of substantive, actionable feedback from the Ambassadors on a variety of topics. The Ambassadors were highly engaged and felt empowered to be guiding clinical research to be more patient-focused and relevant. Importantly, their motivation, while heightened due to their roles as Ambassadors, is still likely reflective and aligned with those of the general ALS population, suggesting that their advice was practical and helpful for the study. Their feedback now forms an instructional library that can be consulted for use in future research initiatives. In addition, some of the ideas and principles from the Ambassadors are pertinent to the current health care situation with COVID-19 and have begun to suggest important changes to potentially help simplify the study procedures for the patients even further.
One of the key changes implemented to the study protocol was a reduction in the number of study visits required. Frequent sample collection was recognized as a significant burden for patients. Equally important was the Ambassadors’ advocation for receiving timely reporting on the results of their clinical assessments, which they believed would motivate further study engagement. Both learnings are consistent with findings from previous patient engagement workshops (Citation5,Citation6). Additionally, the entry criteria were further refined to ensure the enrollment of appropriate patients.
The collaboration with the Ambassadors was instrumental in making the REFINE-ALS study design more patient-centric while maintaining the scientific rigor. The efficient and successful refinement of the REFINE-ALS study is, in itself, a testament to the dedication of ALS-CRLI and its Ambassador program to help support clinical research in this field. Our experience highlights the importance of, and advocates for, gaining and integrating patient perspectives in future clinical research initiatives in ALS.
Acknowledgments
The authors would like to thank the ALS Research Ambassadors for taking part in the advisory board meetings and for providing invaluable feedback that helped in the development of the REFINE-ALS study, and in particular, Kathleen Fowler and Madeline Kennedy, for their contributions to this manuscript. MTPA did not have any input into this report beyond that provided by the present or former MTPA employees listed as authors. p-value communications provided technical writing, editing, and publication assistance and was funded by MTPA.
Declaration of interest
Dr. Berry has performed consulting for MTPA, Denali Therapeutics, and Alexion, and has acted as an investigator or received research funding from Amylyx Pharmaceuticals, Anelixis Pharmaceuticals, Biogen, BrainStorm Cell Therapeutics, Genentech, MT Pharma, ALS Association, Muscular Dystrophy Association, ALS Finding a Cure, and ALS One.
Dr. Bedlack has received research support from ALSA, Cytokinetics, MNDA, Orion, and Ultragenyx and consulting support from ALSA, Biohaven, Biogen, BrainStorm Cell Therapeutics, Cytokinetics, Mallinckrodt, MT Pharma America, ITF Pharma, and New Biotic, Inc.
Dr. Mathews is a consultant for MTPA.
Dr. Agnese is a former employee of MTPA.
Dr. Apple is an employee of MTPA.
Additional information
Funding
References
- Berry J, Brooks BR, Genge A, Heiman-Patterson T, Apple S, Benatar M, Bowser R, et al. Radicava/edaravone findings in biomarkers from ALS (REFINE ALS): interim analysis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:286–7.
- Smith SK, Selig W, Harker M, Roberts JN, Hesterlee S, Leventhal D, et al. Patient engagement practices in clinical research among patient groups, industry, and academia in the United States: a survey. PLOS One. 2015;10:e0140232.
- van den Berg LH, Sorenson E, Gronseth G, Macklin EA, Andrews J, Baloh RH, et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology. 2019;92:e1610–e1623.
- McDermott CJ, Bradburn MJ, Maguire C, Cooper CL, Baird WO, Baxter SK, et al. DiPALS: Diaphragm Pacing in patients with Amyotrophic Lateral Sclerosis – a randomised controlled trial. Health Technol Assess. 2016;20:1–186.
- Sheridan S, Schrandt S, Forsythe L, Hilliard TS, Paez KA. The PCORI engagement rubric: promising practices for partnering in research. Ann Fam Med. 2017;15:165–70.
- Gregg A, Getz N, Benger J, Anderson A. A novel collaborative approach to building better clinical trials: new insights from a patient engagement workshop to propel patient-centricity forward. Ther Innov Regul Sci. 2019; 2168479019849875.
- Bedlack R, Pogemiller A, Shefner J, Cudkowicz M, Heiman-Patterson T. ALS clinical research learning institutes (ALS-CRLI): empowering people with ALS to be research ambassadors. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:216–21.
- Musson LS, McDermott CJ, Hobson EV. Exploring patient and public involvement in motor neuron disease research. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:511–20.
- Sohn E. Fundraising: The Ice Bucket Challenge delivers. Nature. 2017;550:S113–S14.
- Department of Defense – Congresionally Directed Medical Research Programs. FY20 Amyotrophic Lateral Sclerosis Research Program (ALSRP). Available at: https://cdmrp.army.mil/alsrp/default. Accessed June 11, 2020.
- Bedlack RS, Wicks P, Vaughan T, Opie A, Blum R, Dios A, et al. Lunasin does not slow ALS progression: results of an open-label, single-center, hybrid-virtual 12-month trial. Amyotroph Lateral Scler Frontotemporal Degener. 2019; 20:285–93.
