Abstract
Background: Clinical stage in amyotrophic lateral sclerosis (ALS) can be assigned using King’s staging with a simple protocol based on the number of CNS regions involved and the presence of significant nutritional or respiratory failure. It is important that the assigned clinical stage matches expectations, and generally corresponds with how a health care professional would intuitively stage the patient. We therefore investigated the relationship between King’s clinical ALS stage and ALS stage as intuitively assigned by health care professionals. Methods: We wrote 17 case vignettes describing people with ALS at different disease stages from very early limited disease involvement through to severe, multi-domain disease. During two workshops, we asked health care professionals to intuitively stage the vignettes and compared the answers with the actual King’s clinical ALS stage. Results: There was a good correlation between King’s clinical ALS stage and intuitively assigned stage, with a Spearman’s Rank correlation coefficient of 0.64 (p < 0.001). There was no difference in the intuitive stages assigned by practitioners of different types or at different levels of experience. Conclusions: Across a spectrum of ALS scenarios, King’s clinical ALS stage corresponds to intuitive ALS stage as assigned by a range of health care professionals.
Introduction
Amyotrophic lateral sclerosis (ALS), otherwise known as motor neuron disease (MND), is a neurological disease characterized by progressive loss of upper and lower motor neurons, resulting in progressive muscle weakness and eventual paralysis, with death usually resulting from neuromuscular respiratory failure (Citation1,Citation2). Disease duration in ALS varies greatly between individuals (Citation3–6), even though median time to death is just 40 to 44 months from onset (Citation7,Citation8). Disease progression rates range from very aggressive, rapid disease to very slowly progressive disease, and an objective measure of disease progression is needed to guide management and help in research. In cancer, staging is used as an objective indication of disease progress and often informs treatment decisions. Biomarkers of disease stage are being investigated in ALS (Citation9–11), but until a robust biomarker of disease stage is found, clinical staging is an alternative. King’s clinical ALS staging (Citation7) has been proposed for use as a staging method for ALS and has been validated in various studies (Citation12–15), and some biomarkers have been mapped to King’s stage (Citation16–18). Recently, we used King’s staging to estimate the clinical stage at which Riluzole, a treatment for ALS, showed greatest effect (Citation13). The King’s ALS stage corresponds well to anatomical clinical spread, and in this regard differs from the MiToS staging system, which corresponds more to the spread of functional impairment experienced by the patient (Citation19). The King’s ALS clinical staging system is straightforward, using the El Escorial CNS regions (Citation20). The number of involved regions is counted, and the result gives the clinical stage from Stage 1 to 3. If the patient has significant respiratory or nutritional failure (defined within the system) then they are automatically assigned to Stage 4.
It is important that clinical staging matches expectations, and corresponds with how a health care professional would intuitively stage a patient based on their clinical experience with the disease, so that the meaning of any given stage can be easily interpreted. We therefore investigated the relationship between King’s clinical ALS stage and ALS stage as intuitively assigned by health care professionals.
Materials and methods
Clinical vignettes
We wrote 17 case vignettes of patients with ALS, representing different stages of the disease, with Stage 1 (3 vignettes), Stage 2 (7 vignettes), Stage 3 (3 vignettes) and Stage 4 (4 vignettes) represented, as well as a spectrum of cases with bulbar, upper and lower limb involvement, upper and lower motor neuron signs and symptoms, bulbar and spinal site of onset, and cases requiring respiratory support and gastrostomy intervention. In all vignettes, the patients described had a diagnosis of ALS, with clinical examination and investigation consistent with the diagnosis. The vignettes are available in the Supplementary Material.
Participants
Data for analysis were generated from two workshops. The first took place in Milan, Italy, in May 2016, and the second in Ljubljana, Slovenia, in May 2017. The workshops were part of a general clinical trials methods training course (TRICALS) during the annual European Network for the Cure of ALS (ENCALS) meetings and provided training for groups of health care professionals including neurologists, nurses, therapists, and researchers, with varying years of experience in ALS. Participants were asked to intuitively stage the clinical vignettes from Stage 1 (early stage disease) to Stage 4 (late stage disease). They were also asked to provide information about their role, how long they had worked in the ALS field and to provide comments where appropriate if they wished to. Sixty-one participants were classified into three groups in accordance with their role (doctors, nurses, other health care professionals/researchers). Participants were additionally classified based on their work experience into two groups, those with ten years’ experience or more, and those with less than ten years’ work experience. All participants involved provided written consent. This current study does not require ethics approval due to analysis being conducted on fully anonymised data.
Statistical analysis
To measure the reliability of intuitive staging across the entire cohort, we calculated a Spearman’s Rank correlation coefficient between the actual King’s clinical stage represented by each vignette, and stage assessed intuitively. We also calculated Spearman’s Rank correlation coefficients for the different types of health care professional included in the study (doctors, nurses and allied health care professionals), and for those with less than 10 years, or 10 years or greater experience working in ALS. A correlation coefficient is a statistical measure of the strength of the linear relationship between two measurements, in this case King’s ALS staging and intuitive staging. In general, a value above 0.8 (positive or negative) is regarded as a strong correlation, and a value between 0.6 and 0.8 is moderately strong (Citation1,Citation2). Analyses were performed in SPSS v20.0 and GraphPad Prism v6.07.
Results
The study consisted of 61 participants in total, with doctors (65.5%), nurses (9.1%) and other allied health care professionals (25.5%) represented in the cohort. There was an even distribution between those with less than 10 years’ experience working in ALS (50.8%) and those with 10 years’ or greater experience (48.2%).
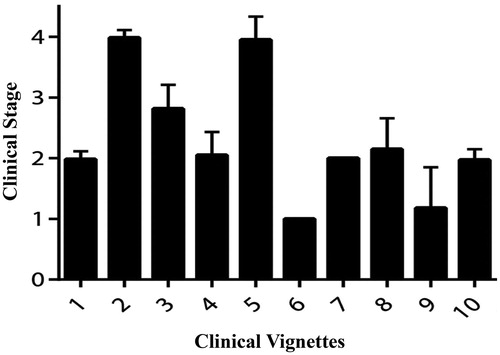
There was a good correlation between stages assigned using King’s clinical staging and stages assigned intuitively by the course participants (Spearman’s Rho = 0.64, p < 0.001). This was true for every health care professional group: doctors (Spearman’s Rho = 0.68, p < 0.001), nurses (Spearman’s Rho = 0.59, p < 0.001) and allied health care professionals (Spearman’s Rho = 0.58, p < 0.001). The same stage as the actual King’s stage was declared intuitively for case vignettes representing King’s Stage 1 by 73.0%, Stage 2 by 53.0%, Stage 3 by 41.1% and Stage 4 by 48.9%. The correlation between staging intuitively and the actual King’s clinical stage was higher for those with at least 10 years' experience working with patients with ALS (Spearman’s Rho = 0.66, p < 0.001) than for those with less than 10 years’ experience (Spearman’s Rho = 0.62, p < 0.001). Overall, in all cases, the most frequent alternative answer for intuitive staging was only one stage different from the actual King’s stage ().
Discussion
We have demonstrated that the King’s clinical staging system correlates well with clinical stage assigned intuitively by health care professionals. It is important that clinical staging matches expectations and corresponds with how a health care professional would intuitively stage a patient based on their clinical experience with the disease, so that the meaning of any given stage can be easily interpreted and so that the system has face validity.
There is currently no validated disease-stage biomarker for ALS. Imaging-based biomarkers are not currently possible, and histopathological biomarkers in life are not feasible. Laboratory biomarker methods are being developed but are still in their infancy. Nevertheless, they hold promise for the future (Citation9,Citation21,Citation22). Until a laboratory-based biomarker is developed, clinical staging is an alternative. Clinical disease staging allows a simple description of the extent of disease progression. Staging systems have been used in cancer to guide patient management and treatment for years, and are designed so as to be intuitively obvious in having higher numbers representing later or more advanced disease.
In ALS, two recent staging systems have been proposed, King’s clinical staging and Milano-Torino staging (Citation7,Citation23). The King’s system is similar to cancer staging in mapping clinical spread with disease progression. Both ALS staging systems are complementary as we and others have previously shown, and are simple to apply (Citation24). This simplicity is important so that all members of the multidisciplinary team can stage uniformly, facilitating discussion within a multidisciplinary team in conjunction with the ALS patient (Citation25). Furthermore, clinical presentation in ALS greatly varies and phenotypes might overlap, so a system that allows uniform and easily understood measurement of disease stage is helpful.
A weakness of this study is the limited number of participants in each subgroup. Although the agreement between actual King’s clinical stage and intuitive staging was high, it was not perfect. However, we found that the limits of agreement lay within a single stage, with systematic biases being negligible, represented equally by cases of over- and under-staging, indicating that the extent of agreement is clinically acceptable. Stage 2 was overrepresented in the clinical vignettes because this is the most likely scenario at diagnosis, but every stage was represented by at least three vignettes. Interestingly, stage 4 was the easiest to intuitively stage. Out of the 61 participants only two did not score this stage correctly. Although the staging criteria included a subdivision into stage 4A and stage 4B when first proposed, they are regarded as a single stage 4 in the current implementation. Of the two people who answered wrongly, one staged a stage 4A vignette wrongly as stage 3 and the other a stage 4B vignette wrongly as stage 3.
The benefits of clinical staging in ALS have been shown in understanding health utility (Citation15,Citation26), mental health (Citation15), cognitive impairment (Citation14,Citation27,Citation28) and health economics (Citation26,Citation29) .Here we show that the system has face validity in corresponding well to the intuitive understanding of the degree to which an individual has progressed. King’s staging is very quick to apply and as such is simple to include prospectively in clinical trials, but it can also be well estimated retrospectively if ALSFRS-R has been collected (Citation30).
Supplemental Material
Download MS Word (25.6 KB)Acknowledgements
We thank the participants involved in the study.
Declaration of interest
AAC is a consultant for Mitsubishi-Tanabe Pharma, GSK, and Chronos Therapeutics, and chief investigator for clinical trials for Cytokinetics and OrionPharma.
Additional information
Funding
References
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–72.
- Al-Chalabi A, Hardiman O, Kiernan MC, Chiò A, Rix-Brooks B, van den Berg LH. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 2016;15:1182–94.
- Pupillo E, Messina P, Logroscino G, Beghi E. Long-term survival in amyotrophic lateral sclerosis: a population-based study. Ann Neurol. 2014;75:287–97.
- Knibb JA, Keren N, Kulka A, Leigh PN, Martin S, Shaw CE, et al. A clinical tool for predicting survival in ALS. J Neurol Neurosurg Psychiatry [Internet] 2016;87:1361–1367.
- Westeneng H-J, Debray TPA, Visser AE, van Eijk RPA, Rooney JPK, Calvo A, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol 2018;17:423–433.
- Westeneng HJ, Al-Chalabi A, Hardiman O, Debray TP, van den Berg LH. The life expectancy of Stephen Hawking, according to the ENCALS model. Lancet Neurol. 2018;17:662–3.
- Roche JC, Rojas-Garcia R, Scott KM, Scotton W, Ellis CE, Burman R, et al. A proposed staging system for amyotrophic lateral sclerosis. Brain. 2012;135:847–52.
- Ferraro D, Consonni D, Fini N, Fasano A, Del Giovane C, Mandrioli J, et al. Amyotrophic lateral sclerosis: a comparison of two staging systems in a population-based study. Eur J Neurol. 2016;23:1426–32.
- Müller H-P, Turner MR, Grosskreutz J, Abrahams S, Bede P, Govind V, et al. A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:570–9.
- Oeckl P, Jardel C, Salachas F, Lamari F, Andersen PM, Bowser R, et al. Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph Lateral Scler Front Degener 2016;17:404–413.
- Turner MR. Progress and new frontiers in biomarkers for amyotrophic lateral sclerosis. Biomark Med. 2018;12:693–6.
- Hardiman , Balendra R, Jones A, Jivraj N, Steen IN, Young CA, et al. Use of clinical staging in amyotrophic lateral sclerosis for phase 3 clinical trials. J Neurol Neurosurg Psychiatry [Internet]. 2015;86:45–9.
- Fang T, Al Khleifat A, Meurgey JH, Jones A, Leigh PN, Bensimon G, et al. Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. 2018;17:416–22.
- Trojsi F, Santangelo G, Caiazzo G, Siciliano M, Ferrantino T, Piccirillo G, et al. Neuropsychological assessment in different King's clinical stages of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:228–35.
- Jones AR, Jivraj N, Balendra R, Murphy C, Kelly J, Thornhill M, et al. Health utility decreases with increasing clinical stage in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener 2014;15:285–91.
- Chen X, Wei QQ, Chen Y, Cao B, Ou RW, Hou Y, et al. Clinical disease stage related changes of serological factors in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener 2019;20:53–60.
- Canosa A, Calvo A, Moglia C, Manera U, Vasta RD, Pede F, et al. Brain metabolic changes across King’s stages in amyotrophic lateral sclerosis: a 18F-2-fluoro-2-deoxy-d-glucose-positron emission tomography study. Eur J Nucl Med Mol Imaging 2020. https://link.springer.com/article/10.1007/s00259-020-05053-w#citeas
- Menon P, Higashihara M, Bos M, Geevasinga N, Kiernan MC, Vucic S. Cortical hyperexcitability evolves with disease progression in ALS. Ann Clin Transl Neurol. 2020;7:733–41.
- Fang T, Al Khleifat A, Stahl DR, Murphy C, LiCALS U-M, Young C. Comparison of the King’s and MiToS staging systems for ALS. Amyotroph Lateral Scler Front Degener 2016;18:227–32.
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8:e75091.
- Chiò A, Calvo A, Bovio G, Canosa A, Bertuzzo D, Galmozzi F, et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA Neurol. 2014;71:1134–9.
- Chiò A, Hammond ER, Mora G, Bonito V, Filippini G. Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:38–44.
- Fang T, Al Khleifat A, Stahl DRL, La Torre C, Murphy C, Young C, et al. Comparison of the King’s and MiToS staging systems for ALS. Amyotroph Lateral Scler Front Degener 2017;18:3–4.
- NICE. Motor neurone disease: assessment and management (NG42). Natl Inst Heal Care Excell Guidel [Internet] 2016;1–48. https://www.nice.org.uk/guidance/ng42
- Moore A, Young CA, Hughes DA. Health utilities and costs for motor neurone disease. Value Heal 2019;22:1257–65.
- Crockford C, Newton J, Lonergan K, Chiwera T, Booth T, Chandran S, et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology [Internet]. 2018;91:e1370–e1380.
- Consonni M, Dalla Bella E, Contarino VE, Bersano E, Lauria G. Cortical thinning trajectories across disease stages and cognitive impairment in amyotrophic lateral sclerosis. Cortex. 2020;131:284–94.
- Schönfelder E, Osmanovic A, Müschen LH, Petri S, Schreiber-Katz O. Costs of illness in amyotrophic lateral sclerosis (ALS): a cross-sectional survey in Germany. Orphanet J Rare Dis. 2020;15:149.
- Balendra R, Jones A, Jivraj N, Knights C, Ellis CM, Burman R, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:279–84.