Abstract
Background: Evidence has accumulated to support the involvement of gastrointestinal (GI) dysfunction, possibly via gut microbial dysbiosis and alterations in the enteric nervous system, in the pathophysiology of different neurodegenerative diseases. However, whether patients with GI dysfunction have altered risk of amyotrophic lateral sclerosis (ALS) remains unknown.
Methods: Based on a historical nationwide cohort study—ESPRESSO—in Sweden, we compared the risk of ALS among individuals with a previous GI biopsy finding of normal mucosa or non-specific inflammation, as two conditions of GI dysfunction, to that of individuals without any GI biopsy. We identified all individuals with a GI biopsy result of either normal mucosa (n = 483,442) or non-specific inflammation (n = 566,663) during 1965–2016 in Sweden as the exposed groups. For each exposed individual, we randomly selected up to five controls from the general Swedish population after individual matching by age and sex. Both the exposed and unexposed individuals were followed from date of biopsy (exposed individuals) or date of selection (unexposed individuals) until ALS diagnosis, emigration out of Sweden, death, or 31 December 2016, whichever came first. Stratified Cox regression models were used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs).
Results: Compared to individuals without GI biopsy, individuals with a GI biopsy result of normal mucosa had an increased risk of ALS (HR = 1.22; 95%CI: 1.04–1.42) after excluding the first 2 years of follow-up to alleviate concern of surveillance bias. This increased risk was noted among male (HR = 1.20; 95%CI: 0.94–1.51) and female (HR = 1.23; 95%CI: 1.01–1.50), as well as among younger (<60 years; HR = 1.17; 95%CI: 0.94–1.44) and older (≥60 years; HR = 1.24; 95%CI: 0.99–1.56) individuals. In contrast, no association was observed for a GI biopsy result of non-specific inflammation (HR = 1.00; 95%CI: 0.88–1.15). Neither of the GI biopsy results was related to the mortality risk after ALS diagnosis.
Conclusions: Individuals with a GI biopsy result of normal mucosa—representing potentially a distinct type of GI dysfunction—had a higher future risk of ALS. No association was however noted for a GI biopsy result of non-specific inflammation. Further studies are needed to validate this finding and to understand the underlying reasons for the contrasting result pattern.
Introduction
With an incidence rate of about 1–2 per 100,000 person-years in most countries, amyotrophic lateral sclerosis (ALS) is a relatively rare but incurable and relentlessly progressive neurodegenerative disease, which is characterized by motor neuron loss in the brain and spinal cord (Citation1). Disease onset occurs commonly in the mid-to-late 60s, and death occurs typically 3–5 years after symptoms onset, most often caused by respiratory paralysis (Citation2). Around 10% of ALS cases are familial, whereas the etiology for patients with sporadic ALS remains elusive (Citation2).
Evidence has accumulated to support the involvement of altered gastrointestinal (GI) function in the pathophysiology of neurodegenerative diseases (Citation3), likely through the gut-brain axis which is jointly mediated by the central nervous system (CNS), the enteric nervous system (ENS), and the intestinal microbiota (Citation4). One hypothesis for the involvement of the gut-brain axis in neurodegenerative diseases is microbial dysbiosis. Gut microbiota is increasingly recognized as contributing to the function of the nervous system (Citation5), through affecting the blood-brain barrier formation, myelination, neurogenesis, and microglia maturation (Citation6). Accumulating evidence has indeed implicated that shifted microbiota composition might be involved in various neurodegenerative diseases, including Parkinson’s disease (PD) (Citation7), Alzheimer’s disease (Citation8), multiple sclerosis (Citation9), and ALS (Citation10). Another hypothesis concerns the pathogenesis of misfolded protein aggregations commonly seen in neurodegenerative diseases (Citation11). For instance, the spread of α-synuclein proteins from the ENS to the CNS has been proposed in PD, with multiple studies demonstrating the presence of α-synuclein aggregates in intestinal biopsies from pre-clinical PD patients (Citation12). Protein aggregates in the intestines might lead to altered GI function, including increased intestinal permeability as shown in patients with PD (Citation13) and a rodent model of ALS (Citation14) as well as altered GI motility which has been proposed as an early pre-motor symptom of PD (Citation15) and ALS (Citation16). A higher risk of PD (Citation17) and dementia (Citation18) has also been suggested among individuals with irritable bowel syndrome.
To this end, by using data from a historical nationwide cohort study, the ESPRESSO Study, in Sweden, we aimed to use a GI biopsy finding of normal mucosa or non-specific inflammation as two indicators for GI dysfunction, which might suggest gut microbial dysbiosis and impaired ENS but not severe inflammations such as inflammatory bowel diseases, and explored their associations with the development and prognosis of ALS.
Methods
Study design
We performed a cohort study based on the ESPRESSO study, which includes histopathology record data concerning the GI tract, liver, gallbladder, and pancreas during 1965–2016 from all 28 pathology departments in Sweden, including biopsy date, topography (where the biopsy was taken), and morphology (biopsy appearance) (Citation19). The Swedish version of the SNOMED system was used to record the morphological type of these biopsies (M codes) (Citation19). In total, there are 2.1 million unique individuals with 6.1 million biopsy records in ESPRESSO. For an individual with biopsy data (i.e. index person), up to five controls were randomly selected from the general Swedish population and individually matched to the index person by age, sex, calendar year of biopsy, and county of residence. The population controls should be living in Sweden and had no biopsy record up to the time of biopsy of the index person. Both the index persons and their matched population controls were then individually followed from date of first biopsy of the index persons until the end of 2016 (for all health outcomes except for death) or the end of 2017 (for death), through cross-linkages to other Swedish national registers, including the Patient Register, the Prescribed Drug Register, the Total Population Register, and the Causes of Death Register, using the individually unique personal identity numbers (Citation19).
Because we were interested in GI dysfunctions that are not due to known inflammations, we determined to study primarily GI biopsy results of normal mucosa and non-specific inflammation in the present study. We firstly included all individuals with a GI biopsy result of normal mucosa (n = 483,442) and their individually matched population controls (n = 2,392,647). We then included all individuals with a GI biopsy result of non-specific inflammation (n = 566,663; M codes: M40000, M40400, M40460, M41000, M42000, M42100, M43000, M45000, and M47000) and their individually matched population controls (n = 2,724,515). To assess the accuracy of the biopsy data, we (JFL) reviewed the biopsy reports from 320 randomly selected individuals with GI biopsy result of normal mucosa (colorectal: n = 160; upper GI: n = 160) and found high positive predictive values of >98% for both upper and lower GI biopsies with a result of normal mucosa (personal communication, April 23, 2020).
All individuals were then followed from cohort entry until a first ALS diagnosis, emigration, death, or 31 December 2016 whichever came first. Emigration out of Sweden was identified from the Total Population Register. Because we were unable to ascertain the outcome (diagnosis of ALS) for individuals that migrated out of Sweden, we used emigration as one of the censoring events. The date of first biopsy was used as the cohort entry for persons with biopsy, whereas the date of selection was used as the cohort entry for the population controls. Individuals with newly diagnosed ALS during follow-up were identified through the Swedish Patient Register, according to the Swedish revisions of the International Classification of Disease codes (ICD-8 348.00, ICD-9 335.2, or ICD-10 G12.2). The date of first hospital visit concerning ALS was used as the date of ALS diagnosis. In a validation study based in Stockholm, we found a > 90% positive predictive value for the diagnosis of ALS based on the Swedish Patient Register (Citation20).
Statistical analyses
Because the cohort participants might receive multiple GI biopsies with different results during the follow-up, we considered all GI biopsies during the follow-up of a specific individual and used the GI biopsy result as a time-varying exposure to minimize information bias (Citation21). For example, the population controls would all first contribute person-time to the “no biopsy” group since cohort entry. Some of them would then contribute person-time to the exposed groups (“normal mucosa” group or “non-specific inflammation” group), if they received a GI biopsy with a result of normal mucosa or non-specific inflammation during follow-up, from the date of biopsy.
We used the stratified Cox regression models, with attained age at time of cohort entry as the underlying time scale, to estimate the hazard ratio (HR) and 95% confidence interval (CI) of ALS in relation to a GI biopsy result of normal mucosa or non-specific inflammation. To alleviate concern of surveillance bias, due for example to the known diagnostic delay of ALS (Citation22), in the main analysis, we excluded the first 2 years of follow-up from the analysis. We also used a lag window of three or four years in the sensitivity analyses to test the robustness of our result. In addition to adjusting for the matching variables including age at time of cohort entry (as underlying time scale), sex, calendar year of biopsy, and county of residence, we also adjusted for the number of healthcare visits from 2 years before to 1 year before biopsy, representing the regular frequency of healthcare visit, to adjust for healthcare seeking behavior. To assess whether the studied associations would differ between male and female, younger and older people, we stratified the analyses by gender and age (<60 years or ≥60 years at cohort entry). To assess whether the associations would differ by location of the biopsy, we also separately examined biopsies in the upper or lower part of the GI tract. As a secondary analysis, we also investigated whether a GI biopsy result of normal mucosa or non-specific inflammation would be related to the survival of ALS patients. We compared therefore age at diagnosis, sex, median survival time, mortality rate, and Kaplan-Meier curves among ALS patients with different GI biopsy results.
Finally, because we had no information on the indications for GI biopsies, we performed an additional sensitivity analysis to investigate all diagnoses in the GI tract during the 5 years before first GI biopsy (index persons) or date of selection (population controls), using information from the Swedish Patient Register. We then used conditional logistic regression to compare the frequencies of different GI diagnoses between the two exposed groups and their respective controls. We then visualized log2 transformed odds ratios (ORs) from these models in a scatter plot.
Data analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and Stata (version 15.0; StataCorp LP, College Station, TX) and R version 3.6.0 (R core team (Citation23)). A two-sided p ≤ 0.05 was considered statistically significant for the primary exposure.
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden.
Results
The mean (standard deviation, SD) age at cohort entry was 44.44 (20.40) years for individuals with a GI biopsy result of normal mucosa and 50.82 (21.83) years for individuals with a GI biopsy result of non-specific inflammation (). During follow-up, a total of 367, 1273, 442 and 2,105 individuals were newly diagnosed with ALS in individuals with a biopsy result of normal mucosa and their matched controls, individuals with a biopsy result of non-specific inflammation and their matched controls, respectively.
Table 1 Characteristics of cohort participants.
In the main analysis, after excluding the first 2 years of follow-up, we observed that, compared with individuals without any GI biopsy, there was an increased risk of ALS among individuals with a GI biopsy result of normal mucosa (HR = 1.22; 95%CI: 1.04–1.42, p = 0.0122) (). The increased risk was noted among male (HR = 1.20; 95%CI: 0.94–1.51) and female (HR = 1.23; 95%CI: 1.01–1.50), as well as among the younger (<60 years at cohort entry; HR = 1.17; 95%CI: 0.94–1.44) and older (≥60 years at cohort entry; HR = 1.24; 95%CI: 0.99–1.56) individuals. Although the increased risk was not statistically significant for the lower GI tract, it was clear for the upper GI tract (HR = 1.25; 95%CI: 1.04–1.50). Similar results were also observed in the sensitivity analyses after excluding the first 3 or 4 years of the follow-up (). In contrast to normal mucosa, no association was noted between a GI biopsy result of non-specific inflammation and ALS risk ().
Table 2 Hazard ratios (HRs) and 95% confidence intervals (CIs) of amyotrophic lateral sclerosis (ALS) among individuals with a gastrointestinal (GI) biopsy result of normal mucosa, compared to matched individuals without any gastrointestinal biopsy.
Table 3 Hazard ratios (HRs) and 95% confidence intervals (CIs) of amyotrophic lateral sclerosis (ALS) among individuals with a gastrointestinal (GI) biopsy result of non-specific inflammation, compared to matched individuals without any gastrointestinal biopsy.
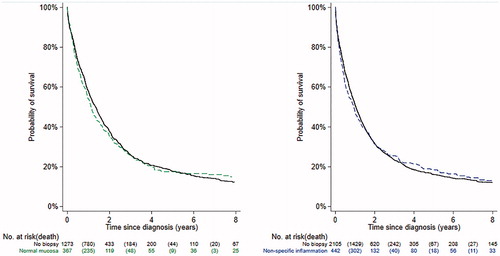
ALS patients with a GI biopsy result of normal mucosa or non-specific inflammation did not differ clearly from ALS patients without any GI biopsy, in terms of age at diagnosis and crude mortality rate (Supplementary Table 1). No significant differences was found in overall survival after ALS diagnosis, when comparing ALS patients with a previous GI biopsy result of normal mucosa (p for log-rank test = 0.9768) or non-specific inflammation (p for log-rank test = 0.9604) to patients without any GI biopsy ().
Figure 1 Kaplan-Meier plots of the probability of surviving patients relative to years since ALS diagnosis; Left figure, individuals with a biopsy result of normal mucosa (green dashed line) and their matched controls (p for log-rank test = 0.9768); Right figure, individuals with a biopsy result of non-specific inflammation (blue dashed line) and their matched controls (p for log-rank test = 0.9604).
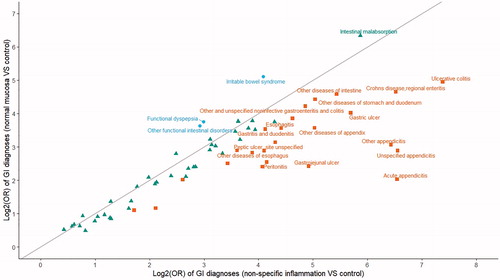
Individuals with a biopsy result of either normal mucosa or non-specific inflammation had clearly increased frequencies of GI diagnoses during the 5 years prior to the first biopsy, compared with their respective controls (). Although individuals with normal mucosa and non-specific inflammation shared many of the increased diagnoses, individuals with normal mucosa were more likely to receive diagnoses suggestive of functional GI symptoms, such as functional dyspepsia, irritable bowel syndrome, intestinal malabsorption, and other functional intestinal disorders, whereas individuals with non-specific inflammation were more likely to receive diagnoses suggestive of inflammatory symptoms, including esophagitis, gastroenteritis, gastritis and duodenitis, gastric ulcer, ulcerative colitis, Crohn’s disease, peritonitis, and appendicitis.
Figure 2 Scatter plot of log2 transformed odds ratios (ORs) of gastrointestinal related diagnoses during the 5 years prior to the first biopsy (individuals with GI biopsy) or date of selection date (population controls). ORs were calculated from conditional logistic regression model, and p-values for all ORs were less than 0.05. Blue circles denote ORs that are greater for normal mucosa than non-specific inflammation. Green triangles denote ORs that are similar between normal mucosa and non-specific inflammation. Brown squares denote ORs that are smaller for normal mucosa than non-specific inflammation.
Discussion
In this population-based cohort study, we explored for the first time the association of gastrointestinal (GI) biopsy results with future risk of ALS. We found that, after using a lag window of two (main analysis) or more (sensitivity analyses) years in the analyses to minimize the influence of surveillance bias, a GI biopsy result of normal mucosa was associated with a higher risk of ALS. In contrast, no clear association was observed between a GI biopsy result of non-specific inflammation and ALS risk. Furthermore, GI biopsy results did not seem to correlate with disease prognosis after ALS diagnosis.
The noted association of a GI biopsy result of normal mucosa with ALS might lend further support to the involvement of altered GI function in the pathophysiology of neurodegenerative diseases, either as a contributory disease mechanism or non-motor prodromal symptoms (Citation3). Different hypotheses have been proposed with the aim to understand the involvement of GI dysfunction in neurodegenerative diseases, including primarily microbial dysbiosis in the gut and aggregations of misfolded proteins in the enteric nervous system (ENS) (Citation3).
Indeed, SOD1 ALS mouse models are shown to have a clear gut dysbiosis and reduced intestinal permeability (Citation24). Germ-free SOD1G93A mice or treating SOD1G93A mice with broad-spectrum antibiotics demonstrate exacerbated disease progression (Citation10). Two potential microbiota-produced disease-modifying metabolites—butyrate and nicotinamide—have been implicated to ameliorate disease progression, through improving gut integrity, restoring gut homeostasis and prolonging life span (Citation14), improving motor symptoms, or restoring gene expression patterns and gene-ontology pathways that are known to be disrupted in ALS (Citation10). Human research using stool samples from ALS patients has however so far yielded inconsistent results. Three studies noted altered microbiota composition in ALS patients, compared with controls (Citation10,Citation25,Citation26), whereas one study showed no substantial alteration when comparing 25 ALS patients with 32 age- and sex-matched healthy controls (Citation27). In a recent study, we observed a dose-response relationship between increasing numbers of antibiotics use, which is known to affect gut microbiota composition and functionality, and increasing risk of ALS (Citation28). The underlying reasons for such inconsistency might include various methodological limitations, including the use of different study populations (ethnicity, dietary habits, etc.), relatively small sample sizes, and varying methods for evaluating microbial communities (16S ribosomal RNA vs metagenomic sequencing). Furthermore, almost all human studies used stool as the sample for gut microbiome analysis, due to its easy access; to what extent the stool microbiome reflects the mucosal microbiome remains however unclear.
Impaired ENS in the gut might be another explanation. Key GI functions including gut motility and permeability, as well as maintenance of the mucosa and mucosal immune responses are all modulated by the ENS (Citation5). Dysfunction of ENS in the GI tract, including diffuse abdominal pain, delayed gastric emptying, constipation, a feeling of fullness, or nausea, has been documented among patients with ALS (Citation29,Citation30). Compared to wild type animals, transactivation response DNA-binding protein 43 (TDP-43) transgenic ALS mice appeared to have more cytoplasmic accumulation of TDP-43 protein in the myenteric plexus (Citation31) and superior mesenteric ganglion (Citation32). Overexpression of TDP-43 protein may lead further to reduced gut motility and other GI dysfunction, which likely occur before the loss of motor neurons in spinal cord becomes evident (Citation31). A GI phenotype has also been reported in transgenic mice that express superoxide dismutase 1 (SOD1) Gly93Ala, the most studied of the SOD1 mutations in ALS (Citation33). No data are however available from the more common forms of genetically caused ALS, including C9orf72. Although data on ALS are relatively scarce, enteric symptoms and pathological protein assembly have been more often reported in Parkinson’s disease and Alzheimer’s disease (Citation34).
In contrast to a GI biopsy result of normal mucosa, a GI biopsy result of non-specific inflammation was not associated with ALS risk. The precise reasons underlying the contrasting results remain unknown. Chance finding may be a possibility, although we had a large-scale nationwide historical cohort study. Validation studies from independent populations are needed as well as studies of other neurodegenerative diseases. If these findings are validated in other studies and for other neurodegenerative diseases, we hypothesize that a GI dysfunction that does not involve severe inflammation or mucosal lesions might be of specific relevance to neurodegenerative diseases including ALS. For instance, such GI dysfunction might involve more impaired ENS function as a result of pathological protein aggregates that are of relevance for the development of neurodegenerative diseases in general. Indeed, in the sensitivity analysis of all diagnoses during the 5 years before GI biopsy, we found that individuals with a GI biopsy finding of normal mucosa had more often diagnoses related to functional GI symptoms (e.g. functional dyspepsia and irritable bowel syndrome), whereas individuals with a GI biopsy finding of non-specific inflammation had more often diagnoses related to inflammatory symptoms (e.g. esophagitis, gastroenteritis, gastric ulcer, ulcerative colitis, Crohn’s disease, peritonitis, and appendicitis). Experimental studies using different disease models are however needed to verify or refute such hypothesis.
This study is characterized by the nationwide population-based cohort design with large sample size as well as complete and long follow-up. The objective and prospective ascertainment of biopsy results and ALS diagnosis from different healthcare registers minimized the potential selection and information biases commonly existent in observational studies. Although it was impossible to verify all the diagnoses of ALS in the study because of the national and historical nature of the study, it has been shown that the positive predictive value of register-based ALS definition is likely satisfactory, with a > 90% positive predictive value according to a validation study conducted in Stockholm (Citation20). Furthermore, our biopsy report validation study found positive predictive values of >98% for both upper and lower GI biopsies with normal mucosa (personal communication, April 23, 2020). Moreover, thanks to the fact that we had complete information on all participants’ entire biopsy history, we considered the GI biopsy result as a time-varying exposure during the entire follow-up, and thereby avoided to the largest extent potential misclassification of exposure.
Nevertheless, this study has several limitations. One limitation is the lack of information on the indications for GI biopsies, which precluded the possibility to explore indications leading to the GI biopsies and ALS risk. Such information might also help to disentangle the contrasting findings between GI result of normal mucosa and non-specific inflammation. In a sensitivity analysis, we found however that patients with a GI biopsy finding of no mucosal lesion were more likely to experience symptoms related to functional GI diagnoses whereas individuals with a GI biopsy of non-specific inflammation were more likely to experience symptoms related to inflammatory GI diagnoses. Second, because of the register-based nature of the study, we had no clinical information on the ALS patients, and could therefore not examine whether the associations would differ for ALS patients of different characteristics. It remains a possibility that patients with GI dysfunctions long before ALS diagnosis might represent a specific subgroup of ALS. Finally, although we matched exposed and unexposed group by age and sex individually and adjusted for calendar period, county of residence and healthcare seeking behavior, residual confounding remains a concern. For instance, the lack of information on potential risk or protective factors for ALS (e.g. smoking, body mass index) precluded the possibility to further explore the role of such factors in the reported associations.
In conclusion, we found that a GI biopsy of normal mucosa– representing potentially a distinct type of GI dysfunction—was associated with an increased future risk of ALS. No association was however observed for a GI biopsy result of non-specific inflammation. Further studies are needed to validate this finding and to understand the underlying reasons for the contrasting result pattern.
Supporting information
Supplementary table 1. Prognosis of patients with amyotrophic lateral sclerosis (ALS), by different results of gastrointestinal biopsy.
Supplemental Material
Download MS Word (14.4 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Data availability statement
Due to legal and ethical reasons, the data cannot be shared.
Additional information
Funding
References
- Taylor JP, Brown RH Jr., Cleveland DW. Decoding ALS: from genes to mechanism. Nature 2016;539:197–206.
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–72.
- Ambrosini YM, Borcherding D, Kanthasamy A, Kim HJ, Willette AA, Jergens A, et al. The gut-brain axis in neurodegenerative diseases and relevance of the canine model: a review. Front Aging Neurosci. 2019;11:130.
- Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94.
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell 2016;167:915–32.
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016;167:1469–80 e12.
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–55.
- Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015.
- Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019;572:474–80.
- Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349:1255555.
- Brundin P, Ma J, Kordower JH. How strong is the evidence that Parkinson’s disease is a prion disorder? Curr Opin Neurol. 2016;29:459–66.
- Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord. 2018;50:104–7.
- Zhang Y-G, Wu S, Yi J, Xia Y, Jin D, Zhou J, et al. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther. 2017;39:322–36.
- Gao X, Chen H, Schwarzschild MA, Ascherio A. A prospective study of bowel movement frequency and risk of Parkinson’s disease. Am J Epidemiol. 2011;174:546–51.
- Piccione EA, Sletten DM, Staff NP, Low PA. Autonomic system and amyotrophic lateral sclerosis. Muscle Nerve. 2015;51:676–9.
- Lai SW, Liao KF, Lin CL, Sung FC. Irritable bowel syndrome correlates with increased risk of Parkinson’s disease in Taiwan. Eur J Epidemiol. 2014;29:57–62.
- Chen CH, Lin CL, Kao CH. Irritable bowel syndrome is associated with an increased risk of dementia: a nationwide population-based study. PLoS One. 2016;11:e0144589.
- Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–14.
- Mariosa D, Hammar N, Malmström H, Ingre C, Jungner I, Ye W, et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol. 2017;81:718–28.
- Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087.
- Longinetti E, Regodon WA, Samuelsson K, et al. The Swedish motor neuron disease quality registry. Amyotroph Lateral Scler Frontotemporal Degener 2018;19(7-8): 528–537.
- R Core Team (2020). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- Wu S, Yi J, Zhang YG, Zhou J, Sun J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep. 2015;3:e12356.
- Rowin J, Xia Y, Jung B, Sun J. Gut inflammation and dysbiosis in human motor neuron disease. Physiol Rep. 2017;5:e13443.
- Fang X, Wang X, Yang S, Meng F, Wang X, Wei H, et al. Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front Microbiol. 2016;7:1479.
- Brenner D, Hiergeist A, Adis C, Mayer B, Gessner A, Ludolph AC, et al. The fecal microbiome of ALS patients. Neurobiol Aging. 2018;61:132–7.
- Sun J, Zhan Y, Mariosa D, Larsson H, Almqvist C, Ingre C, et al. Antibiotics use and risk of amyotrophic lateral sclerosis in Sweden. Eur J Neurol. 2019;26:1355–61.
- Vucic S. Sensory and autonomic nervous system dysfunction in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 2017;43:99–101.
- Baltadzhieva R, Gurevich T, Korczyn AD. Autonomic impairment in amyotrophic lateral sclerosis. Curr Opin Neurol. 2005;18:487–93.
- Esmaeili MA, Panahi M, Yadav S, Hennings L, Kiaei M. Premature death of TDP-43 (A315T) transgenic mice due to gastrointestinal complications prior to development of full neurological symptoms of amyotrophic lateral sclerosis. Int J Exp Pathol. 2013;94:56–64.
- Hatzipetros T, Bogdanik LP, Tassinari VR, Kidd JD, Moreno AJ, Davis C, et al. C57BL/6J congenic Prp-TDP43A315T mice develop progressive neurodegeneration in the myenteric plexus of the colon without exhibiting key features of ALS. Brain Res. 2014;1584:59–72.
- Kaur SJ, McKeown SR, Rashid S. Mutant SOD1 mediated pathogenesis of amyotrophic lateral sclerosis. Gene 2016;577:109–18.
- Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–28.
