Abstract
Objective: To investigate the role of arterial blood gas (ABG) analysis parameters (blood carbon dioxide, pCO2; oxygen, pO2; carbonate, HCO3−; standard base excess, SBE) in monitoring respiratory function and ventilation compliance after noninvasive mechanical ventilation (NIV) adaptation, predicting survival in ALS patients. Methods: We selected the first ABG performed after NIV start in ALS patients followed from 2000 to 2015 in Turin ALS Center. Correlations between ABG parameters and survival were calculated. Risk for death/tracheostomy was computed at modifying ABG parameters by using Cox regression models, adjusted for the main prognostic factors. Kaplan–Meier curves were then performed and compared. Results: A total of 186 post-NIV ABGs were included. HCO3− and SBE showed a significant correlation with survival after NIV (respectively, R = −0.183, p = 0.018 and R = −0.200, p = 0.010). Risk for death/tracheostomy after NIV was significantly higher at increasing HCO3− and SBE blood levels, especially when HCO3− was >29 mmol/L and SBE >4 mmol/L (respectively, HR 1.466, 95% CI 1.068–2.011, p = 0.018 and HR = 1.411, 95% CI 1.030–1.32, p = 0.032). Survival in NIV was higher in patients with HCO3− < 29.0 mmol/L and SBE < 4.0 mmol/L. Conclusions: HCO3− and SBE blood levels are markers of ventilation compliance, tolerance and efficacy, being able to predict survival after NIV start in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder affecting motor neurons. A critical aspect of the disease is respiratory failure due to respiratory muscles weakness that occurs during the course of the disease.
Noninvasive mechanical ventilation (NIV) is recognized as the treatment of choice for impending symptoms and signs of respiratory failure, improving patients’ quality of life and survival (Citation1). Patients’ compliance to NIV and adequate ventilator settings are crucial for improving long-term NIV efficacy (Citation2,Citation3).
In this study, we aimed at investigating the role of arterial blood gas (ABG) parameters, such as blood carbon dioxide (pCO2), oxygen (pO2), carbonate (HCO3−), and standard base excess (SBE), in monitoring respiratory function after NIV adaptation and predicting patients’ survival.
Materials and methods
Data collection
All clinical records of ALS patients included in the Piemonte and Valle d’Aosta ALS Registry (PARALS) and diagnosed from 2000 to 2015 in Turin ALS Center were checked. We collected a dataset including the ABGs performed after NIV start. For patients who performed more than one post-NIV ABG, we selected the first one for our analysis. ABGs were performed in the morning through radial arterial puncture by a trained nurse or pulmonologist and immediately analyzed using blood gas analyzer, routinely calibrated, as appropriate.
Patients with severe medical conditions or with signs of uncompensated acidosis/alkalosis (ABG pH <7.35 and >7.45) were excluded from the analysis. For each patient, we collected age at onset, sex, site of onset, date of diagnosis, date of death/tracheostomy, date of ABG, and date of ALSFRS-r scale. Survival was assessed from the date of ABG to death/tracheostomy or censoring date (31st December 2018). The disease progression rate (ΔALSFRS) was computed as the ratio between the difference 48—ALSFRS-r at the time of ABG and the time interval in months between onset and the day of ABG. Bulbar involvement at time of ABG was derived from the scores in the first three ALSFRS-r scale items. It was classified as absent if all three items had a score equal to 4, moderate if at least one of them had a score equal to 3 or 2, severe if at least one of them had a score equal to 1 or 0.
We considered as NIV-treated all the patients who were prescribed NIV and used it for at least 1 day (intention-to-treat analysis) (Citation4). NIV prescription was always done by an expert pulmonologist, who followed the most updated clinical practice guidelines (Citation5–7).
Statistical analysis
Differences of discrete and continuous variables were analyzed using the χ2 test and Student’s t test or Wilcoxon rank-sum and Mann–Whitney U tests, respectively. A p value <0.05 was considered significant. Correlations between ABG parameters (pCO2, pO2, HCO3−, SBE) and disease duration from date of ABG were calculated using the non-parametric two-tailed Spearman’s rank correlation test. In order to check a significant increase of risk of death/tracheostomy, we assessed the association between ABG values and survival, by using Cox proportional hazards models, adjusted for sex, age at onset, bulbar involvement at time of ABG and ΔALSFRS. Kaplan–Meier curves were then performed and compared with log rank test. Data were analyzed using IBM SPSS Statistics for Windows, Version 26.0. IBM Corp. Released 2019.
Standard protocol approvals, registrations, and patient consents
The study design was approved by the Ethical Committee of the Azienda Ospedaliero-Universitaria Città della Salute of Turin (Prot. N. 0036344).
Results
A total of 186 post-NIV ABGs were included in our analysis. Descriptive statistics of patients are shown in . Median time interval between NIV start and ABG was 32.0 days (IQR 14.0–92.3).
Table 1 Descriptive statistics (n = 186 patients).
Among ABG parameters, HCO3−, SBE, and pH showed a significant correlation with survival on NIV (respectively R = −0.183, p = 0.018 for HCO3−; R = −0.200, p = 0.010 for SBE; for pH R = −0.175, p = 0.024) as shown in .
Table 2 Correlations between ABG parameters and survival from date of post-NIV ABG.
Risk for death/tracheostomy in NIV was significantly higher at increasing HCO3− and SBE blood levels (respectively, for HCO3− HR 1.055, 95% CI 1.017–1.095, p = 0.004; for SBE HR = 1.060, 95% CI 1.020–1.101, p = 0.003, ). In particular when HCO3− levels were higher than 29 mmol/L and SBE exceeded 4 mmol/L a significant increase for death/tracheostomy was found (for HCO3− HR 1.466, 95% CI 1.068–2.011, p = 0.018; for SBE HR = 1.411, 95% CI 1.030–1.32, p = 0.032, ).
Table 3 Risk for death/tracheostomy from date of post-NIV ABG.
Table 4 Risk for death/tracheostomy from date of post-NIV ABG.
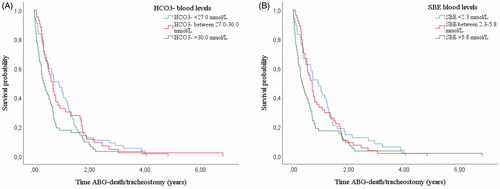
When grouping patients in tertiles according to HCO3− and SBE levels, patients included in the first and in the second tertile showed a longer survival in NIV than patients in the last tertile ()).
Figure 1 Kaplan–Meier curves: survival from time of post-NIVABG. Patients (n = 160) grouped according to HCO3− blood levels (a) and SBE blood levels (b). Both patients with HCO3− and SBE blood levels in the first and second tertile showed a longer survival than patients in the last tertile. In particular patients with HCO3− < 27.0 mmol/L and between 27.0 and 30.0 mmol/L showed a longer survival than patients with HCO3− > 30.0 mmol/L (respectively, 0.80 years, IQR 0.32–1.41 versus 0.32 years, IQR 0.10–0.72; p = 0.035 and 0.60 years, IQR 0.29–1.66 vs. 0.32 years, IQR 0.10–0.72; p = 0.041). Patients with SBE < 2.3 mmol/L and between 2.3 and 5.8 mmol/L showed a longer survival than patients with SBE > 5.8 mmol/L (respectively, 0.89 years, IQR 0.29–1.40 vs. 0.30 years, IQR 0.08–0.69; p = 0.028 and 0.61 years, IQR 0.30–1.37 vs. 0.30 years, IQR 0.08–0.69; p = 0.046).
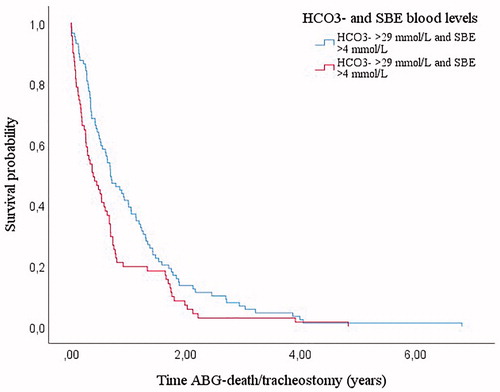
Survival in NIV was longer in patients with HCO3− blood levels <29.0 mmol/L and SBE <4.0 mmol/L ().
Figure 2 Kaplan–Meier curves: survival from time of post-NIVABG according to HCO3− and SBE cutoffs (n = 160). Patients with HCO3− <29.0 mmol/L and SBE <4.0 mmol/L showed a longer survival than patients with both parameters increased (0.69 years, IQR 0.33–1.37 vs. 0.37 years, IQR 0.12–0.77; p = 0.013).
Post-NIV ABG parameters did not show any significant difference between patients with bulbar and spinal onset and between patients with and without bulbar involvement at time of NIV (). Bulbar onset did not influence survival in NIV (respectively for bulbar onset HR 1.145, 95% CI 0.812–1.614, p = 0.440), while a severe bulbar involvement at time of NIV was associated with an increased risk for death/tracheostomy during ventilation (HR 1.949, 95% CI 1.109–3.425, p = 0.020).
Table 5 Comparison of post-NIV ABG parameters between patients with bulbar and spinal onset and between patients with and without bulbar involvement during ventilation.
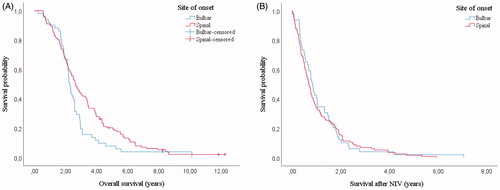
Although patients with bulbar onset showed a lower overall survival than patients with spinal onset (2.28 years, IQR 1.84–2.95 vs. 2.65 years, IQR 1.72–4.35, p = 0.041, ) their survival after the starting of NIV was not different (0.82 years, IQR 0.39–1.53 vs 0.67, IQR 0.32–1.47, p = 0.675, ).
Figure 3 Kaplan–Meier curves: survival in bulbar onset and spinal onset patients (n = 160). Overall survival was lower for bulbar patients (2.28 years, IQR 1.84–2.95 vs. 2.65 years, IQR 1.72–4.35, p = 0.041, a) but survival in NIV was not statistically different (0.82 years, IQR 0.39–1.53 vs. 0.67, IQR 0.32–1.47, p = 0.675, b).
Discussion
We investigated the role of ABG parameters after NIV start as predictor of both patients’ adherence to ventilatory therapy and survival. We found that after NIV start pCO2 levels were not correlated to survival in ALS patients, and only HCO3− and SBE levels were predictive of death/tracheostomy. Specifically, when HCO3− blood levels were above 29 mmol/L and SBE above 4 mmol/L risk for death/tracheostomy was increased by more than 40% and survival significantly shortened.
The correlation between ABG parameters and survival was found to be stronger before NIV starting (Citation8): this could be due to the real efficacy of ventilatory therapy, that can normalize pCO2 and HCO3− blood levels if performed correctly, thus reducing the strength of correlation between ABG values and survival. The inverse correlation between pH and survival could be related to the chronic HCO3− increase in ALS patients with nocturnal hypoventilation (Citation9); so a higher pH is a sign of chronic compensation during a respiratory failure of longer duration and thus a marker of worse prognosis in ALS. Indeed acidosis is not a common event in our patients, being usually associated with an acute respiratory failure concomitant to pneumonia or other precipitant causes (Citation10).
In a recent international survey about the monitoring of respiratory function among ALS tertiary centers, 37.8% (17/45) of European respondents reported that they always/nearly always use ABG, compared to only 1.4% (1/69) of US counterparts (Citation11). In a study evaluating the role of ABG in predicting respiratory function and the necessity to start NIV, we found that when pCO2 was >42 mmHg, HCO3− >26 mmol/L, or SBE >2 mmol/L there was a significant decline in FVC and an increase of risk for death/tracheostomy (Citation8). In addition, an isolated increase of HCO3−/SBE blood levels turned out to be a marker of nocturnal hypoventilation (NH), a frequently asymptomatic entity that prompts to start NIV.
The last European Federation of Neurological Societies (EFNS) guidelines recommend using FVC regularly for respiratory monitoring before and after NIV adaptation, while SNIP is recommended for bulbar patients (Citation7). The National Institute for Care and Excellence (NICE) guidelines suggest performing respiratory function tests (oxygen saturation, FVC, SNIP/MIP) every 2–3 months and ABG analysis only in case of desaturation (Citation12). About the role of ABG in post-NIV respiratory management, the American Academy of Neurology (AAN) guidelines mentioned only the importance of hypercapnia (pCO2 > 50 mmHg) to indicate invasive ventilation starting (Citation6). The German National Guideline for Treating Chronic Respiratory Failure guidelines for chronic respiratory failure indicate to monitor pCO2 at least 1–2 times per year after NIV starting, as the gold standard to monitor ventilatory therapy adherence (Citation13), even if values of daytime pCO2 cannot rule out NH, particularly in neuromuscular patients (Citation14).
Based on our results, we showed that ABG is a useful tool to monitor mechanical ventilation compliance, and can predict patients’ survival. HCO3−/SBE levels are strictly related to patients’ adherence and tolerance to ventilatory support; therefore, clinicians should pay attention when their blood levels are increased, even with pCO2 normal values. Indeed, progressive respiratory decompensation in ALS patients leads to chronic respiratory acidosis that causes a compensatory increase in serum bicarbonate levels (Citation15). A scarce adaptation/answer to NIV could lead to a nocturnal increase of pCO2 and HCO3−/SBE levels, but in the morning when ABG is usually performed, it is still possible to detect an increase in HCO3−/SBE levels without a parallel increase of pCO2, since HCO3−/SBE levels do not decreases fast as pCO2 levels with daytime orthostatic breathing. Consequently, finding high HCO3−/SBE levels even without hypercapnia should prompt the modification of ventilation settings, for example changing the type of mask or ventilation parameters, or increase in the duration of NIV usage. Adjusting NIV parameters may reduce nocturnal desaturations and improve prognosis (Citation16,Citation17).
Moreover, we found that, even if patients with bulbar onset show a lower overall survival than patients with spinal onset, their survival after the starting of NIV does not differ. Indeed, ABG parameters during ventilation did not differ between bulbar and spinal onset patients. Only the presence of a severe bulbar involvement at time of ventilation turned out to be a negative prognostic factor. This finding confirms that an intensive educational training and adaptation to NIV in a multidisciplinary setting increases compliance and tolerance also in patients with bulbar impairment (Citation3). Therefore, even if bulbar impairment remains a negative prognostic factor for ALS and a lower percentage of bulbar onset patients are able to adapt to ventilation, partly due to the higher prevalence of cognitive dysfunction in bulbar patients (Citation18,Citation19), NIV shows a similar benefit in all disease phenotypes.
Our study has some limitations. First, due to its retrospective nature, we could not compare ABG values to other pulmonary function tests, such as forced vital capacity (FVC), nocturnal oximetry or mechanical ventilator’s recordings to confirm our data on NIV compliance. Second, due to the intrinsic difficulty to perform a complete neuropsychological evaluation in ALS patients with advanced disease, we could not include cognitive evaluation in our study.
Due to the importance of respiratory support, ventilation adherence is crucial in ALS patients: ABG evaluation, especially considering SBE and HCO3− blood levels, turned to be an inexpensive and informative tool to assess patients’ compliance to NIV, to monitor pulmonary function, and to modify ventilator settings when necessary, in order to improve both quality of life and survival.
Author contributions
Dr Manera has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Manera, Torrieri, Calvo, Chiò. Acquisition, analysis, or interpretation of data: Manera, Torrieri, Moglia, Canosa, Vasta, Palumbo, Solero, Ribolla, Mattei, Mora, Calvo, Chiò. Drafting of the manuscript: Manera, Torrieri. Critical revision of the manuscript for important intellectual content: Manera, Torrieri, Moglia, Mattei, Mora, Calvo, Chiò. Statistical analysis: Manera, Torrieri. Obtained funding: Moglia, Mattei, Calvo, Chiò. Administrative, technical, or material support: Canosa, Vasta, Palumbo, Solero, Ribolla. Supervision: Mattei, Mora, Calvo, Chiò.
Acknowledgments
Our special thanks also to our colleagues Dr Giuseppe Tabbia, Dr Marco Michele Bardessono, Paola Calvi, Dr Fulvia Ribolla, Dr Luana Focaraccio, Dr Elena Rindone, Dr Michela Bellocchia, Dr Cinzia Ferrero, Dr Nicola Launaro, Dr Enio Mantellini, Dr Biagio Polla, Dr Alessandro Mastinu, Andrea Tagliabue, and Sandro Longu for their valuable collaboration in ALS patients management.
Declaration of interest
Dr Manera, Dr Torrieri, Dr Moglia, Dr Canosa, Dr Vasta, Dr Palumbo, Dr Solero, Dr Ribolla, Dr Mattei and Dr Mora report no conflicts of interest. Prof Calvo has received research grant from Cytokinetics. Prof Chiò serves on scientific advisory boards for Mitsubishi Tanabe, Roche, Biogen, Denali Pharma, and Cytokinetics.
The sponsor organizations had no role in data collections and analysis and did not participate to writing and approving the manuscript. The information reported in the manuscript has never been reported elsewhere.
Data availability statement
Data will be available upon request by interested researchers.
Additional information
Funding
References
- Dorst J, Ludolph AC. Non-invasive ventilation in amyotrophic lateral sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419857040.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–7.
- Volanti P, Cibella F, Sarvà M, De Cicco D, Spanevello A, Mora G, et al. Predictors of non-invasive ventilation tolerance in amyotrophic lateral sclerosis. J Neurol Sci. 2011;303:114–8.
- Chiò A, Calvo A, Moglia C, Gamna F, Mattei A, Mazzini L, et al.; PARALS. Non-invasive ventilation in amyotrophic lateral sclerosis: a 10 year population based study. J Neurol Neurosurg Psychiatry. 2012;83:377–81.
- Miller RG, Rosenberg JA, Gelinas DF, Mitsumoto H, Newman D, Sufit R, et al. Practice parameter: the care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology: ALS Practice Parameters Task Force. Neurology 1999;52:1311–23.
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al.; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1218–26.
- Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al.; EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)-revised report of an EFNS task force . Eur J Neurol. 2012;19:360–75.
- Manera U, Torrieri MC, Moglia C, Viglione M, Daviddi MAR, Matteoni E, et al. The role of arterial blood gas analysis (ABG) in amyotrophic lateral sclerosis respiratory monitoring. J Neurol Neurosurg Psychiatry. 2020;91:999–1000.
- Hadjikoutis S, Wiles CM. Venous serum chloride and bicarbonate measurements in the evaluation of respiratory function in motor neuron disease. QJM. 2001;94:491–5.
- Mayaux J, Lambert J, Morélot-Panzini C, Gonzalez-Bermejo J, Delemazure J, Llontop C, et al. Survival of amyotrophic lateral sclerosis patients after admission to the intensive care unit for acute respiratory failure: an observational cohort study. J Crit Care. 2019;50:54–8.
- Heiman-Patterson TD, Cudkowicz ME, De Carvalho M, Genge A, Hardiman O, Jackson CE, et al. Understanding the use of NIV in ALS: results of an international ALS specialist survey. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:331–41.
- National Institute for Health and Care Excellence. (2019). Motor neurone disease: assessment and management (NICE Guideline No. 42). Retrieved from https://www.nice.org.uk/guidance/NG42.
- Windisch W, Geiseler J, Simon K, Walterspacher S, Dreher M, on behalf of the Guideline Commission. German national guideline for treating chronic respiratory failure with invasive and non-invasive ventilation: revised edition 2017 - part 1. Respiration. 2018;96:66–97.
- Ergan B, Oczkowski S, Rochwerg B, Carlucci A, Chatwin M, Clini E, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J. 2019;54:1901003.
- Qureshi M, Shui A, Dibernardo AB, Brown RH, Jr, Schoenfeld DA, Cudkowicz ME. Medications and laboratory parameters as prognostic factors in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:369–74.
- Gonzalez-Bermejo J, Morelot-Panzini C, Arnol N, Meininger V, Kraoua S, Salachas F, et al. Prognostic value of efficiently correcting nocturnal desaturations after one month of non-invasive ventilation in amyotrophic lateral sclerosis: a retrospective monocentre observational cohort study. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:373–9.
- Georges M, Attali V, Golmard JL, Morélot-Panzini C, Crevier-Buchman L, Collet JM, et al. Reduced survival in patients with ALS with upper airway obstructive events on non-invasive ventilation. J Neurol Neurosurg Psychiatry. 2016;87:1045–50.
- Schreiber H, Gaigalat T, Wiedemuth-Catrinescu U, Graf M, Uttner I, Muche R, et al. Cognitive function in bulbar- and spinal-onset amyotrophic lateral sclerosis. A longitudinal study in 52 patients. J Neurol. 2005;252:772–81.
- Chiò A, Moglia C, Canosa A, Manera U, Vasta R, Brunetti M, et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 2019;93:e984–94.
