?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction: Respiratory tests are fundamental for monitoring respiratory function in ALS, and essential in clinical trials. Slow vital capacity (SVC) was canceled in some countries to prevent COVID-19 transmission. We aimed to test phrenic nerve motor responses as an option to SVC in clinical trials. Methodology: Patients followed-up in our unit were selected respecting inclusion criteria used elsewhere: possible/probable/definite disease; onset-age 18–80years; disease duration from disease duration ≤24months; body mass index (BMI)>20kg/m2; respiratory subscore of the revised ALS functional rating scale (ALSFRS-R)≥11; upright SVC ≥ 70%. We added normal phrenic responses (meanPhrenAmpl, ≥0.4mV). All patients were on riluzole. SVC and meanPhrenAmpl were recorded at study entry (T0) and 24 weeks later (T1). Decays were determined. Sample size was calculated for a treatment effect of 30% on the decay rate. Results: We included 317 ALS patients (191 males, 225 spinal-onset), mean onset-age 59.9 ± 10.7 (31–80)years, mean onset BMI 25.48 ± 3.2 (20.1–35)kg/m2, mean disease duration 10.5 ± 5.6 (1–24)months, mean ALSFRS-R 41.54 ± 4.3 (22–47) and respiratory subscore 11.83 ± 0.38 (11–12). MeanPhrenAmpl and SVC were weakly but significantly correlated at T0 and T1. At T1, MeanPhrenAmpl decayed 16.94 ± 16.45% and SVC 13.5 ± 16.86%. For the proposed drug effect, 174 and 272 patients would be needed to recruit using respectively meanPhrenAmpl and SVC decline as the primary outcome measurement (accepting no dropouts). Discussion: Contrary to SVC, meanPhrenAmpl is non-volitional and not associated with aerosolization risk. Lower recruitment number (98 patients less) would be needed, translating shorter inclusion period, trial length and costs, and probable lower missed data rate. MeanPhrenAmp is an alternative test in ALS clinical trials.
Introduction
COVID-19 pandemic has risen many unthinkable difficulties in the medical and research fields, in particular affecting patients with chronic diseases who need regular follow-up, as patients with amyotrophic laterals sclerosis (ALS) (Citation1). Technical procedures were restructured or eventually canceled to prevent the virus transmission (Citation2). ALS patients using ventilatory support and with suspected COVID-19 infection are recommended to use face mask interfaces without expiratory valve, in addition to filters intercalated in the system. Forced (FVC) and slow (SVC) vital capacity, maximal pressures, as well as peak expiratory flow and peak cough flow, are volitional respiratory function tests (RFT) frequently used to monitor the respiratory function in ALS (Citation3–6), which have been canceled in many ALS centers due to the risk of virus transmission (Citation7–8). The respiratory tests are fundamental to monitor the respiratory function in ALS, indicating the need for ventilatory support (Citation3). In addition, their values are used as inclusion criteria in trials, and their results accepted as a primary outcome measure (Citation9).
The diaphragmatic motor responses by transcutaneous phrenic nerve stimulation is a noninvasive, non-volitional test that uses surface electrodes to record the diaphragmatic response (Citation10). Contrary to the volitional RFT, it is usually performed without asking the patient for any specific maneuver, being the stimulus given at the end-expiration phase to guarantee a consistent maximal relaxation of the diaphragm (Citation11). Previous studies have shown that the responses by phrenic nerve stimulation are reliable (Citation12), symmetrical in ALS (Citation13), correlated with FVC (Citation14), predictive of survival (Citation15), and hypoventilation both in spinal and bulbar ALS patients (Citation10), as well of functional status (Citation16). It bypasses the major limitation that FVC/SVC and other RFT face when assessing ALS patients, which is the orofacial paresis (Citation10). As a non-volitional test that does not originate aerosol particles, it can present itself as an alternative to FVC/SVC during the COVID-19 pandemic.
With the present study we aimed to determine if the phrenic nerve motor responses are appropriate as inclusion criterion and outcome measure in clinical trials as compared with SVC.
Methodology
Patients with possible, laboratory-supported probable (Awaji guidelines), probable or definite ALS in accordance with the revised El Escorial criteria and with follow-up in our ALS center in Lisbon were considered. The patients were followed prospectively (before the COVID-19 pandemic), but the analyses was done retrospectively.
Inclusion criteria required, following recent criteria in clinical trials (Citation9): age at onset between 18 and 80 years, disease duration from disease duration at inclusion ≤ 24 months; body mass index (BMI) > 20kg/m2; respiratory subscore of the revised ALS functional rating scale (ALSFRS-R) ≥ 11; upright SVC ≥70% of the predicted value; mean peak-to-peak amplitude of the bilateral phrenic motor responses ≥0.4mV. All patients were stable on riluzole 50mg bid, and none had been included in any trial. We excluded patients with clinical signs of dementia, with other forms of motor neuron disease (primary lateral sclerosis, progressive muscular atrophy, flail-arm, flail-leg, Kennedy’s disease), and with other medical disorders including asthma, chronic obstructive pulmonary disease, diabetes, heart failure, or polyneuropathy.
Slow vital capacity evaluation
SVC was determined with the patients in the sitting position, by using a computer-based USB spirometer (microQuark®, Cosmed®) or standard Jager equipment (two Jäger® Masterlab®, and one Jäger® Masterscreen®, Erich Jager, GmbH, Wurzburg, Germany). All measurements were performed by one of the authors (SP), using microQuark®, Cosmed®, and the same technician for the Jäger equipment. The best of three satisfactory and consistent expiratory maneuvers, each obtained after a maximal inspiratory effort, was used to determine the values of SVC. Predicted values (%) were used for statistical analysis.
Phrenic nerve evaluation
Diaphragmatic compound muscle action potential (CMAP) was elicited bilaterally by percutaneous bipolar electrical phrenic nerve stimulation at the neck (posterior to the middle lateral border of the sternocleidomastoid muscle). Recordings used surface electrodes (with filter setting 20–10 kHz) with the active electrode (G1) positioned at the homolateral costosternal angle and the reference electrode (G2) at the costal margin 16 cm from the active electrode, as described elsewhere (Citation10–15); responses were recorded at the end of expiration to assure diaphragm relaxation (Citation16). Brachial plexus responses were avoided by repositioning the stimulating electrode. A minimum of three consistent motor responses were recorded from each side and the response with the highest peak-to-peak amplitude was selected. The mean peak-to-peak amplitude value (meanPhrenAmpl) of both right- and left-side responses was used for the analyses, considered as an inclusion criterion and follow-up outcome. A cutoff equal or above 0.4mV was chosen as inclusion criterion for normality, as mentioned before in the literature (Citation10).
Statistical analysis
The mean percentage of decay between baseline and week 24 and standard deviation (SD) were calculated for both SVC and PhrenAmpl. Value at baseline was normalized to 100%. If there was no decay the variation was considered as 0%. Demographics of the population included, Pearson’s correlations between studied variables, Student’s t differences between decays were calculated using SPSS®24 IBM®. A p < 0.05 was considered as significant.
The sample size was considered as the minimum number of patients needed to complete a 24-week randomized parallel controlled trial with 80% power to detect a difference of 30% in the decay rate (p < 0.05) between the placebo and treatment arms, to be in line with recent clinical trials (Citation9). Values were pooled using the percentage of mean and SD decays for SVC and meanPhrenAmpl in the recruited population, considered as the placebo arm, with a two-tailed alpha of 0.05. No additional patients were considered to compensate for drop-outs.
To calculate the number of patients we applied the formula (Citation17):
Results
We included 317 ALS patients (191 males), with mean age at disease onset of 59.9 ± 10.7 (31–80) years, mean BMI at disease onset of 25.48 ± 3.2 (20.1–35) and mean disease duration at study entry of 10.5 ± 5.6 (1–24) months. The region of onset was spinal in 225, and bulbar in 92 (29%). Mean ALSFRS-R at study entry was 41.54 ± 4.3 (22–47) and mean RofALSFRS-R was 11.83 ± 0.38 (11–12).
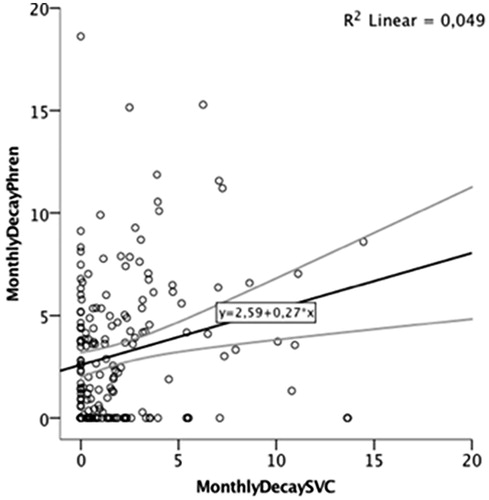
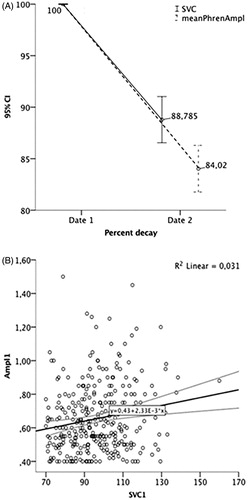
At study entry, meanPhrenAmpl (0.65 ± 0.20mV, range 0.40–1.50mV, ) was weakly but significantly correlated with SVC (96.18 ± 15.2%, range 70–160%, ), r = 0.18, p = 0.002 (). MeanPhrenAmpl decayed 3.6 ± 4.0%/month and 16.94 ± 16.45%/24 weeks, while SVC decayed 2.4 ± 3.15%/month and 13.5 ± 16.86%/24 weeks (). A significant weak correlation between monthly decay of the two variables was found (r = 0.22, p = 0.003, ).
Figure 1 (A) MeanPhrenAmpl at study entry and 6 months after (values are presented in mV); (B) SVC at study entry and 6 months after (values are presented in percentage of the predicted value).
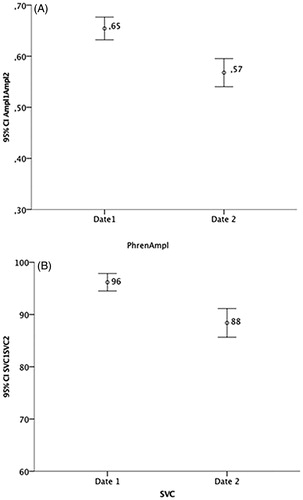
Figure 3. Percentage of SVC and MeanPhrenAmpl decay at month 6 (A) and correlation between both variables at month 6 (B).
A number of 174 patients would be necessary to recruit using meanPhrenAmpl decay as the primary outcome, and 272 using mean SVC decline as the primary outcome measurement.
Discussion
In this study supported by data from a population of ALS patients eligible for a clinical trial and considering respiratory function as the main treatment outcome (Citation9), phrenic nerve responses at baseline and its monthly decay were significantly but weakly correlated with SVC values. The weak correlation found can be explained by the different physiological nature of the tests, since phrenic nerve responses give specific information on the diaphragmatic strength, while SVC mainly evaluates the expiratory function but is also dependent on a full inspiration (Citation5).
Using phrenic nerve amplitude as an inclusion criterion required the recruitment of a lower number of patients (98 patients less). This would lead to shorter inclusion periods, shorter length duration of the studies and lower costs regarding time and resources. Taking into account that phrenic nerve studies is a non-volitional test, not dependent on bulbar function or cognition, we could anticipate a lower rate of missing values. Moreover, when the respiratory function is poor, recording motor responses from the diaphragm is feasible (Citation10), and the results are symmetric (Citation13), reliable (Citation12,Citation14), reproducible (Citation18), with an intra-rater variability of 10% in healthy subjects and also in patients with primary lateral sclerosis (Citation12), and a test tolerability close to 100% in our experience. Its values decay overtime (Citation14,Citation18), but are feasible until there is marked respiratory involvement and intolerance to supine position.
There has been a clear growth in the number of clinical trials in ALS for the last 25 years, which have explored new outcomes for testing compound’s effect. Survival has been used as the primary outcome in many ALS trials. However, it depends on life-extending interventions and gives poor information about functionality. Moreover, survival is not the ideal solution for exploratory clinical trials since many patients are recruited, and trials are lengthy and expensive (Citation19).In some trials ALSFRS-R is preferred as the primary outcome, reducing trial duration and sample size. However, ALSFRS-R does not follow a linear decline, depends on patient judgment, and is influenced by cognitive function (Citation20). RFT are critical in ALS, since the respiratory function (as evaluated by FVC/SVC, and maximal pressures) is a major prognostic factor (Citation21). Some trials have selected FVC/SVC as the primary outcome, according to drug action and the importance of respiratory function (Citation9). Respiratory function involvement has also been used as an inclusion criterion in different trials (Citation5,Citation22). SVC was used in our study, although FVC has traditionally been used to assess respiratory function in ALS. SVC and FVC have been shown to be strongly correlated (Citation23) and similarly predictive of survival (Citation24) and functionality (Citation25) in ALS. However, the results of the conventional RFT depend on the bulbar function, cognition and cooperation of patients (Citation5). In addition, in situations like the COVID-19 pandemic, it is challenging to maintain regular RFT evaluation. In our center, phrenic nerve responses are regularly assessed in ALS patients and gain particular relevance in those with moderate-severe orofacial paresis, cognitive involvement or unable to cooperate with the respiratory manoeuvers. A cutoff of 0.4mV has previously been determined as the lower limit of normality for the peak-to-peak amplitude of the diaphragmatic responses by phrenic nerve stimulation (Citation10). We use this cutoff as a good indicator of diaphragmatic weakness and to start NIV in addition to the presence of respiratory symptoms and the other respiratory measurements (Citation3), being a very decisive factor in the presence of orofacial paresis and poor cooperation. The COVID-19 pandemic is also a unique situation during which phrenic nerve studies can continue being performed without the risk of aerosolization. The same stands true for nocturnal pulsed oximetry, which can complement the information whenever needed. In the design of early-phase trials in ALS, the introduction of predictive or prognostic biomarkers is an important step (Citation22). In this regard, to explore phrenic nerve study as a surrogate marker of respiratory function in ALS seems a good option.
We should be cautious on our results because sample size calculation depends on a large array of assumptions. Inter-rater reliability and the expected number of patients to be lost during follow-up were, for example, not considered in our study. Anyhow, in any trial, a previous training on the measurements used is a rule. Standard operating procedures (SOPs) should be detailed, addressing technical issues, such as type of electrodes, filters, stimulus parameters, as well as the correct positioning of the electrodes, stimulus timing, identification of artifacts, correct recording of the motor responses and selection of the best consistent responses. SOPs, training and intra-rater and inter-rater variability studies have been carried out for other neurophysiological techniques, namely for motor unit number index (MUNIX) (Citation26), which could be easily adapted and replicated for the phrenic studies.
We conclude that phrenic nerve study should be considered in future trials as an option for the inclusion and follow-up of ALS patients.
Declaration of interest
The authors reported no potential conflict of interest.
Additional information
Funding
References
- Andrews JA, Berry JD, Baloh RH, Carberry N, Cudkowicz ME, Dedi B, et al. Amyotrophic lateral sclerosis care and research in the USA during the COVID-19 pandemic: challenges and opportunities. Muscle Nerve. 2020;62:182–6.
- Pinto S, Silani V, Chiò A, Calvo A, Salachas F, Camu W, et al. on behalf of the ENCALS and EAN expert panel. Amyotrophic Lateral Sclerosis and COVID-19: Recommendations to patients and caregivers. 2020. https://www.eanpages.org/2020/04/15/amyotrophic-lateral-sclerosis-and-covid-19-recommendations-to-patients-and-caregivers/
- Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)-revised report of an EFNS task force. Eur J Neurol. 2012;19:360–75.
- Pinto S, de Carvalho M. Breathing new life into treatment advances for respiratory failure in amyotrophic lateral sclerosis patients. Neurodegener Dis Manag. 2014;4:83–102.
- De Carvalho M, Swash M, Pinto S. Diaphragmatic neurophysiology and respiratory markers in ALS. Front Neurol. 2019;10:143.
- Lechtzin N, Cudkowicz ME, de Carvalho M, Genge A, Hardiman O, Mitsumoto H, et al. Respiratory measures in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:321–30.
- Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol-generating procedures and risk of transmission of acute respiratory infections: a systematic review. CADTH Technol Overv. 2013;3:e3101.
- McCormack MC, Kaminsky DA. Pulmonary function laboratories: advice regarding COVID-19. Available from: www.thoracic.org/professionals/clinical-resources/disease-related-resources/pulmonary-function-laboratories.php
- Andrews JA, Cudkowicz ME, Hardiman O, Meng L, Bian A, Lee J, et al. VITALITY-ALS, a phase III trial of tirasemtiv, a selective fast skeletal muscle troponin activator, as a potential treatment for patients with amyotrophic lateral sclerosis: study design and baseline characteristics. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:259–66.
- Pinto S, Turkman A, Pinto A, Swash M, de Carvalho M. Predicting respiratory insufficiency in amyotrophic lateral sclerosis: the role of phrenic nerve studies. Clin Neurophysiol. 2009;120:941–6.
- Miranda B, Pinto S, de Carvalho M. The impact of spasticity on diaphragm contraction: electrophysiological assessment. Clin Neurophysiol. 2018;129:1544–50.
- Torrieri MC, Miranda B, Gromicho M, Pinto S, de Carvalho M. Reliability of phrenic nerve conduction study: in healthy controls and in patients with primary lateral sclerosis. Clin Neurophysiol 2020; 131(5):994–999.
- Pinto S, de Carvalho M. Symmetry of phrenic nerve motor response in amyotrophic lateral sclerosis. Muscle Nerve. 2010;42:822–4.
- Pinto S, Geraldes R, Vaz N, Pinto A, de Carvalho M. Changes of the phrenic nerve motor response in amyo-trophic lateral sclerosis: longitudinal study. Clin Neurophysiol. 2009;120:2082–5.
- Pinto S, Pinto A, de Carvalho M. Phrenic nerve studies predict survival in amyotrophic lateral sclerosis. Clin Neurophysiol. 2012;123:2454–9.
- Miranda B, Gromicho M, Pereira M, Pinto S, Swash M, de Carvalho M. Diaphragmatic CMAP amplitude from phrenic nerve stimulation predicts functional decline in ALS. J Neurol. 2020;267:2123–9.
- Matthews JN. Introduction to randomized controlled clinical trials. London: Arnold (Holder Headline Group); 2000.
- Jenkins JAL, Sakamuri S, Katz JS, Forshew DA, Guion L, Moore D, et al. Phrenic nerve conduction studies as a biomarker of respiratory insufficiency in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:213–20.
- van Eijk RP, Eijkemans MJ, Rizopoulos D, van den Berg LH, Nikolakopoulos S. Comparing methods to combine functional loss and mortality in clinical trials for amyotrophic lateral sclerosis. Clin Epidemiol. 2018;10:333–41.
- Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:414–25.
- Schmidt EP, Drachman DB, Wiener CM, Clawson L, Kimball R, Lechtzin N. Pulmonary predictors of survival in amyotrophic lateral sclerosis: use in clinical trial design. Muscle Nerve. 2006;33:127–32.
- Van Den Berg LH, Sorenson E, Gronseth G, Macklin EA, Andrews J, Baloh RH, et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology. 2019;92:e1610–e1623.
- Pinto S, de Carvalho M. Correlation between forced vital capacity and slow vital capacity for the assessment of respiratory involvement in amyotrophic lateral sclerosis: a prospective study. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:86–91.
- Pinto S, de Carvalho M. Comparison of slow and forced vital capacities on ability to predict survival in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:528–33.
- Pinto S, de Carvalho M. SVC is a marker of respiratory decline function, similar to FVC, in patients with ALS. Front Neurol. 2019;28:109.
- Christoph N, Burkhardt C, Alix J, Castro J, de Carvalho M, Gawel M, et al. Quality control of Motor Unit Number Index (MUNIX) measurements in 6 muscles in a single-subject “round-Robin” setup. PLoS One. 2016;11:e0153948.