Abstract
Objective: To summarize applications of muscle magnetic resonance imaging (MRI) in cross-sectional assessment and longitudinal monitoring of motor neuron diseases and evaluate associations with clinical assessment techniques.
Methods: PubMed and Scopus were searched for research published up to May 2021 relating to muscle MRI in motor neuron diseases, according to predefined inclusion and exclusion criteria. Studies were systematically appraised for bias and data were extracted for discussion.
Results: Twenty-eight papers met inclusion criteria. The studies assessed muscle T1- and T2-weighted signal, diffusion, muscle volume, and fat infiltration, employing quantitative, qualitative, and semi-quantitative approaches. Various regions of interest were considered; changes in thigh and calf muscles were most frequently reported. Preliminary evidence of concordance between clinical and radiological findings and utility as an objective longitudinal biomarker is emerging.
Conclusion: Muscle MRI appears a promising objective, versatile, and practical biomarker to assess motor neuron diseases.
Introduction
Magnetic resonance imaging (MRI) is an established biomarker for many neuromuscular disorders (Citation1–4). However, the role of muscle MRI in motor neuron disease (MND) research and clinical practice remains unclear, and no literature reviews have focused on this topic to date. MND is clinically heterogenous in terms of anatomy, relative upper and lower motor neuron burden, genetics, pathophysiology, and prognosis. Therefore, objective biomarkers of disease progression are necessary to facilitate clinical trials.
Current biomarkers for MND each have limitations. Biometric markers such as forced vital capacity (Citation5) rely on patient technique and effort, and interpretation may be challenging in patients with bulbar or cognitive dysfunction. Functional rating scales (Citation6) are subject to assessor interpretation and may be influenced by symptomatic treatment. Cerebrospinal fluid and serum biomarkers such as neurofilament (Citation7,Citation8) and TDP-43 (Citation9) probe pathophysiology, but utility in longitudinal assessment remains uncertain (Citation10). Neuroradiological techniques accurately exclude ALS “mimics”, but have limited sensitivity at an individual level and correlations with clinical measures are variable (Citation10). Neurophysiological techniques such as motor unit number index (MUNIX) (Citation11) and electrical impedance myography (Citation12) are emerging, but a limited number of muscles can be assessed. Muscle MRI represents an objective, noninvasive and effort-independent tool, and multiple metrics such as muscle volume, fat fraction (FF), and T1/T2 signal characteristics are available to assess pathology.
This review summarises muscle MRI applications in MND (particularly the most common form, amyotrophic lateral sclerosis (ALS)), and other diseases of the motor neuron, namely spinal muscular atrophy (SMA) and spinal and bulbar muscular atrophy (SBMA).
Materials and methods
The PubMed and Scopus databases were searched for literature published up to May 2021 with no filters applied. The search strategy was as follows:
Title: ALS OR MND OR amyotrophic lateral sclerosis OR motor neuron disease OR motor neuron disease OR Lou Gehrig's disease OR spinal muscular atrophy OR spinal bulbar muscular atrophy OR spinal and bulbar muscular atrophy OR SMA OR SBMA OR Kennedy's disease
AND (title/abstract/keywords): MRI OR magnetic resonance imaging
AND (title/abstract/keywords): Muscle OR muscular
To ensure no omissions, the search of PubMed was then repeated with the “muscle OR muscular” term replaced with “NOT brain” and “NOT spinal cord”.
Grey-research databases and search engines were also searched for relevant material. Clinical trials in progress in the registry “ClinicalTrials.gov” were assessed.
Results were screened by one reviewer (AK). Inclusion criteria were primary research papers reporting applications of muscle MRI in MND/ALS, SMA or SBMA in humans. Animal studies, case reports, non-motor neuron diseases research, non-MRI imaging studies, and non-English language studies were excluded. Google Scholar and reference lists were used to perform forward and backward citation searching on all included studies.
Summary data from identified manuscripts were manually extracted into tables, including disease studied, participant numbers, and clinical, MRI, and neurophysiological data measurement techniques. Results in three categories of interest were reported: differences in muscle MRI findings between patients and controls, correlations between muscle MRI and clinical/neurophysiological assessment techniques, and longitudinal changes in muscle MRI measures.
Studies were appraised for bias using both the National Institute of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies (Citation13), and the Newcastle-Ottawa Scale (NOS) for cohort (Citation14) and cross-sectional (Citation15) studies. The threshold for adequacy of follow-up of cohorts in the NOS was set at 20%. To satisfy case ascertainment criteria, MND diagnoses required application of either El-Escorial (Citation16) or Awaji-Shima (Citation17) criteria, and SMA/SBMA diagnoses required genetic confirmation, except in papers published before availability. Criteria relating to multiple assessments of exposure, exposures varying in amount, and non-respondents were not applicable to these observational studies and were omitted.
The terms “qualitative”, “quantitative” and “semi-quantitative” MRI assessment techniques were defined as follows: Qualitative methodologies require subjective judgment and produce non-continuous data, for example, graded analog scales (such as the Mercuri scale). Quantitative techniques are objective methodologies producing continuous data, directly reflecting tissue characteristics, for example, T2 relaxometry, diffusion, volumetrics, or fat fraction. Semi-quantitative techniques are objective methodologies producing continuous data that indirectly reflect inherent tissue characteristics, necessitating expression relative to a reference region.
Results
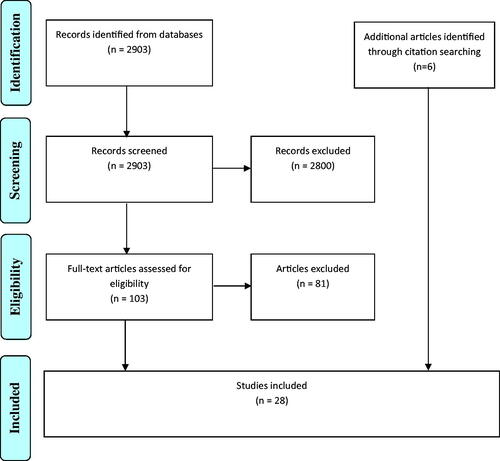
After removal of duplicates, the initial search strategy returned 2903 results (PubMed = 2166, Scopus = 737), of which 2800 were excluded based on screening. Eighty-one further papers were excluded for failure to meet inclusion criteria, and a total of 22 papers were included (see ). Additionally, six further eligible papers identified through citation searching were included, for a final total of 28 papers. Four relevant ongoing clinical trials were identified but not included in this review as none had published preliminary results (NCT04691011, NCT04262570, NCT04690998, NCT02044029).
Among these 28 articles, there were 14 MND/ALS (Citation18–31), 13 SMA (Citation32–44) and three SBMA (Citation20,Citation29,Citation45) patient groups. There were eight multiple-region (Citation21,Citation24–26,Citation28,Citation29,Citation37,Citation45), 13 lower-limb (Citation19,Citation20,Citation32–36,Citation38,Citation40–44), one paraspinal (Citation30), three bulbar (Citation18,Citation22,Citation23) and three whole-body (Citation27,Citation31,Citation39) studies. These papers are summarized in and .
Table 1 Studies included in a literature review.
Table 2 MRI acquisition sequences.
Assessment of bias
The risk of bias tools revealed a generally robust study design. Frequent issues identified were omission of a control group (Citation32,Citation33,Citation36,Citation37,Citation39–41,Citation43), lack of blinding (Citation18–20,Citation23,Citation31,Citation34–36,Citation39–43,Citation45) and inadequate ascertainment of disease status (Citation23,Citation25,Citation40). Only four studies justified the sample size (Citation22,Citation27,Citation31,Citation38). More than 20% cohort attrition was reported in 5/9 longitudinal studies, typical in MND research. Five studies explicitly reported adjustments made for confounding factors (Citation27,Citation32,Citation33,Citation35,Citation42) and none adjusted for disease duration, potentially significant in the context of degenerative diseases. Studies generally performed well on participant recruitment (e.g. selecting representative population samples), evidence of reliable measurement, and outcome reporting. No issues relating to conflicts of interest or funding sources were identified. Overall risk of bias was determined to be low/medium; results are reported in .
Table 3 Assessment of bias using the NHLBI quality assessment tool.
Table 4 Assessment of bias in cohort studies using the NOS.
Table 5 assessment of bias in cross-sectional studies using the NOS.
MND
The first application of muscle MRI in MND was in 1989 when tongue morphological changes were investigated in 16 ALS patients and 20 disease controls (Citation18). Abnormalities of size, shape, position, structure, and signal intensity were seen in ALS patients, who had significantly smaller tongues (9.99 mg vs. 13.3 mg, p < .001) and weaker grip strength (21.8 lb vs. 47.6 lb, p < .001) than disease controls.
Nine years later, the first longitudinal muscle MRI study in MND/ALS investigated 11 ALS patients and eight healthy controls, with follow-up at four months (Citation19). Lower-limb muscle volume and quantitative T1 and T2 relaxation time (T1r/T2r) measurements were compared with compound muscle action potential (CMAP), and maximal voluntary isometric contraction (MVIC). Only mean T2r was significantly different between cohorts at baseline (41.4msec vs. 33.4msec, p = .009), with no longitudinal radiological changes evident. At baseline, MVIC correlated with T2r (r=–0.926, p = .001) and tibialis anterior (TA) volume (r = 0.777, p = .005) but not with T1r. CMAP and T2r also correlated at baseline (r=–0.903, p = .001) and longitudinally (r=–0.63, p = .037). This study provided a solid foundation for quantitative muscle MRI as a potential biomarker in MND/ALS.
In 2013, a longitudinal pilot study demonstrated the stability of muscle volume in 11 healthy control subjects (<5% variability over 12 months) and found trends toward volume decrements in the thenar eminence (–26.84%, p = .056) and TA (–8.29%, p = .077) in four ALS patients (Citation24). The interpretation was however limited by the small sample size and clinical heterogeneity.
Subsequently, nerve and muscle abnormalities were reported in a 2015 cross-sectional cohort of 60 ALS and 8 multifocal motor neuropathy (MMN) patients who underwent MRI of the brachial or lumbosacral plexus (Citation25). MRI abnormalities were more frequent in ALS patients (57% vs. 33%) but did not correlate with muscle strength. Two years later, multiple MRI parameters were assessed in the limb-girdle muscles of 23 ALS and 12 healthy subjects (Citation26). Alterations in T2-weighted and short-tau inversion recovery (STIR) signal intensities were seen in subscapularis and supra/infraspinatus, with fat infiltration and atrophy evident on T1 in all patients, but not controls.
The first whole-body muscle MRI application in MND was reported in 2018, with further longitudinal follow-up in 2020 (Citation27,Citation31). Whole-body semi-quantitative T2-weighted scans were acquired in 29 MND patients (26 ALS, three progressive muscular atrophy (PMA)), and 22 age/gender-matched healthy controls. At baseline, MND patients demonstrated 18% higher whole-body relative T2 signal vs. controls (p < .01), with per-muscle differences ranging from 0.6% in right trapezius to 71.4% in left TA. Relative T2 changes over 4 months were significant only in bilateral TA (p < .017) and not in the tongue, biceps brachii or paraspinal, with no changes found in controls (Citation27). At 12 months, relative T2 signal increased significantly in several lower-limb muscles (bilateral quadriceps, hamstrings, TA, and gastrocnemius/soleus, and right psoas) and also in right first dorsal interosseous (1DIO). Whole-body diffusion-weighted MRI demonstrated no changes. The researchers reported numerous associations between relative T2 signal and Medical Research Council (MRC) muscle strength scores, dynamometry, MUNIX and CMAP (Citation27). In 2018, another small study investigating diffusion-weighted MRI of the tongue in two ALS and five healthy control subjects found decreased fractional anisotropy (FA), increased mean diffusivity (MD), and fewer connecting muscle fibers in patients compared to controls (Citation23).
In 2019, qualitative muscle MRI findings were assessed in 22 ALS, 8 MMN and 15 healthy participants. ALS patients demonstrated significantly more muscle edema and atrophy than the other cohorts (Citation21). Another 2019 study assessed 68 muscles in ten ALS patients and nine healthy controls (Citation28). Overall, muscle atrophy and fatty infiltration were more common in ALS patients, the latter differing significantly from controls in iliopsoas (p = .046), and anterior, (p = .020) and posterior calf (p = .047) muscles, but not in thoracic paraspinal, gluteus maximus, thigh, or hand muscles. Atrophy and fatty infiltration in dominant hand muscles correlated inversely with the ALS functional rating scale revised (ALSFRSr) hand function scores (r=–0.72, p = .01), and associations between these MRI measurements and electromyography (in 1DIO, paraspinal and TA) and MRC scores (in 1DIO and TA) were evident. The authors concluded that electromyography appeared more sensitive for quantification of muscle damage, with radiological techniques preferred to study deeper, inaccessible muscles. A 2020 paper by the same group found that ALS patients and disease controls could be differentiated using MRI of paraspinal muscles, and differences in longissimus dorsi distinguished between bulbar and spinal-onset ALS patients (Citation30).
Finally, in 2020, tongue volume and mean T1 intensity were assessed in large cohorts of 185 MND patients and 104 healthy controls (Citation22). Tongue T1 intensity was lower in bulbar-onset than limb-onset patients (p < .001) and correlated with ALSFRSr bulbar sub-scores (r=–0.2, p = .02), and larger or more ellipsoidal tongues at baseline predicted a slower decline in ALSFRSr. There were however no significant differences in MRI measures between patients and controls.
SMA
The first published study of muscle MRI in SMA, in 1992, described patterns of lower-limb muscle involvement in 17 biopsy-proven SMA patients (3 severe-type, 9 intermediate-type, 5 mild-type) (Citation41). Phenotypes demonstrated distinct radiological signatures, but all exhibited increased subcutaneous fat and preservation of adductor longus. Another small SMA study, from 2004, qualitatively assessed thigh muscle MRI in 8 patients with a rare, genetically unclassified SMA phenotype (Citation36). Subjects were selected on clinical grounds, such as talipes at birth, predominant lower-limb involvement, and signs of neurogenic muscle damage. An increased fat: muscle ratio, diffuse atrophy, and selective sparing of adductor longus and semitendinosus were seen.
In 2011, a comprehensive quantitative protocol measuring thigh muscle volume, percentage muscle, normal signal volume, and normal signal percentage was applied to 14 genetically-confirmed SMA patients (1 SMA I, 6 SMA II and 7 SMA III). Researchers found multiple correlations between MRI and clinical-electrophysiological measures including CMAP, motor unit number estimation (MUNE), dynamometry and functional scales (Citation32). In the same year, this group also published the first longitudinal SMA study, assessing 11 genetically-confirmed patients (4 SMA II and 7 SMA III) (Citation33). Muscle volume remained relatively stable over six months and corroborated previous clinico-radiological associations. Limitations included a lack of control groups and significant heterogeneity between SMA subgroups.
In 2017, fatty infiltration was graded qualitatively in 25 genetically-confirmed SMA IIIb patients (Citation37). Selective involvement of iliopsoas, triceps brachii and quadriceps was evident radiologically, with comparable findings on MRC scores. In the same year, investigators assessed longitudinal changes in quantitative T2 signal, FF and cross-sectional area (CSA) in thigh muscles of 18 genetically-confirmed SMA III patients and 19 healthy controls (Citation35). Significant between-group differences were found in all MRI measures, but there were no longitudinal changes. However, most MRI measures demonstrated excellent reliability (intra-class correlation coefficient >0.9) and correlated well with clinical assessments and age.
In 2019, fat infiltration of lower-limb muscles was assessed in 31 genetically-confirmed SMA patients (10 SMA II, 11 SMA IIIa, 10 SMA IIIb) and 31 healthy controls using T2-weighted MRI, as part of a neurography study (Citation38). SMA subtype dictated infiltration severity, which was particularly marked in types II and IIIa, with no associations found between CMAP and T2-weighted muscle MRI data.
In 2020, muscle involvement patterns in 36 pediatric and 19 adult cases of SMA (18 SMA II, 12 SMA IIIa, 25 SMA IIIb) were investigated using T1-weighted MRI (Citation40). Selective involvement of the glutei and anterior and posterior thigh, and preservation of the medial thigh was again seen, and there were inverse correlations between the Hammersmith functional motor scale expanded (HFMSE) and fatty infiltration (r = 0.69, p < .01) and total atrophy (r = 0.59, p = .01) scores.
Two studies assessing SMA patients taking nusinersen were published in 2020/21. The first investigated whole-body qualitative T1-weighted MRI and lower-limb quantitative diffusion tensor imaging (DTI) changes in two siblings with molecularly-confirmed SMA IIIb (Citation39). No T1 signal changes were observed but DTI demonstrated increased number, length, and organization of muscle fiber tracts at follow-up. The second study assessed thigh FF and quantitative water-T2 signal in three female genetically-confirmed SMA IIIa patients and 11 healthy controls (Citation44). Mean FF in patients increased by 6.7% and the average water-T2 signal decreased by 4.7%. Despite small sample sizes, these studies provided proof-of-concept for muscle MRI applications in therapeutic trials. In 2021, fatty infiltration and atrophy were qualitatively assessed in 20 genetically-confirmed SMA IV patients (Citation34). Relative preservation of tibialis posterior, fibular and extensor digitorum longus, and sparing of the medial thigh compartment was seen. Atrophy and fatty infiltration scores correlated with clinical measures including walking speed, ALSFRSr, HFMSE, and disease duration.
In 2020/2021, FF, T2 relaxometry, and DTI were assessed in leg muscles of 31 genetically-confirmed SMA patients (15 SMA II, 7 SMA IIIa, 9 SMA IIIb) and 20 healthy controls (Citation42), with ten patients completing one-year follow-up (Citation43). At baseline, patients exhibited higher FF (+40%, p < .001), lower MD (1.13 vs. 1.47, p < .001), higher FA (0.41 vs. 0.24, p < .001) and mean T2r differences (p < .001) when compared to controls. Functional scale and MRC scores correlated with FF, MD, and FA but not T2r. At follow-up, there were no significant changes in clinical measurements, but mean thigh FF increased by 1.3%, with a small change in T2r, becoming significant after exclusion of adductor longus and biceps femoris (–0.4ms, p = .02). Limitations of this study included increased fat fractions potentially influencing T2 relaxation results, and no follow-up of controls.
SBMA
The first SBMA muscle MRI study, in 2004, involved one healthy control, three genetically-confirmed SBMA and two ALS patients (Citation20). SBMA patients exhibited involvement of vastus lateralis and the posterior thigh and preservation of gracilis and sartorius. Quantitative fat infiltration and functional remaining muscle area (FRMA) were assessed by another group in lower-limb and bulbar muscles of 21 ALS, 21 genetically-confirmed SBMA and 16 healthy control subjects (Citation29). SBMA and ALS functional rating scores correlated with FF and FRMA, and significant differences were found between patient cohorts and controls for FF.
In 2019, muscle FF was assessed in 40 genetically-confirmed SBMA patients and 25 healthy controls (Citation45). Researchers found increased muscle fat in patients’ paraspinal (12%), thigh (22%), calf (22%), upper arm (12%), and forearm (11%) muscles (all p < .05). Quantitative FF correlated with peak torque (r > 0.68, p < .0001), functional rating score (r = 0.86, p < .0001), walking speed (r = 0.79, p < .0001) and forced vital capacity (r = 0.66, p < .0001).
Discussion
Studies in motor neuron diseases have applied quantitative (Citation18,Citation19,Citation22–24,Citation29,Citation31–33,Citation35,Citation39,Citation42–45), qualitative (Citation18,Citation20,Citation21,Citation25,Citation26,Citation28–30,Citation32–34,Citation36–41,Citation45), and semi-quantitative (Citation27,Citation31) techniques, often in combination, and imaging protocols vary between studies. Most qualitative studies used the five-point Mercuri scale, which grades change from an early moth-eaten appearance to muscle replacement by connective tissue and fat (Citation46). Other applied scales include the modified Goutallier (Citation47), Fischer (Citation48) and Stramare (Citation49) classifications. Quantitative measurements included FF, FRMA, diffusion, volumetrics and relaxometry. A consensus on optimal muscle MRI methodology is yet to emerge.
Whole-body MRI maximizes anatomical coverage but, to date, has necessitated qualitative (Citation39) or semi-quantitative (Citation27,Citation31) methodologies, which have limitations (Citation50). Results from these studies and others assessing isolated regions-of-interest suggest that leg muscle changes appear more easily detectable, likely for technical reasons rather than disease effects. Future research determining the most sensitive and specific protocols to detect muscle damage would enable more standardized approaches, which would facilitate meta-analyses. Preliminary evidence suggests that MRI techniques assessing T2 tissue metrics, FF and FRMA appear particularly sensitive to change in motor neuron diseases. Volumetric studies detect atrophy but there may be clinico-radiological lag. Diffusion-based sequences have not yet demonstrated clear utility, but more research is needed. While qualitative methodologies have informed muscle involvement patterns, objective and quantitative approaches appear more suited candidate biomarkers for future clinical trials.
Associations between clinico-electrophysiological measures and muscle MRI across numerous studies suggest that clinically relevant pathophysiological loss of motor units is reflected. However, clinico-radiological associations were not the primary outcome of any study, and further research is needed.
Identification of selective muscle damage patterns in SMA/SBMA has facilitated diagnosis, with high levels of concordance between studies. However, in ALS, there appears greater clinical heterogeneity, and variance in outcome measurements limits comparisons between studies.
In conclusion, muscle MRI appears a promising tool to study muscle pathophysiology in motor neuron diseases, but further research is required to define its place in clinical and research practice. Changes in patients compared to healthy controls are evident at the group level, but there is individual variability and relatively few studies of disease controls. Group-level clinical and electrophysiological associations suggest that radiological changes in muscle reflect clinically relevant loss of motor units, and longitudinal studies suggest that muscle MRI may represent an objective measure of disease changes. However, the clinical importance of individual-level changes in longitudinal studies remains to be determined, as detected changes have sometimes been subclinical. Some muscles appear more sensitive for the detection of radiological change than others, which has implications for clinical trial applications. Future studies are necessary to investigate the role of fully quantitative, anatomically comprehensive muscle MRI protocols in longitudinal assessment, within achievable timeframes.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Morrow JM, Sinclair CDJ, Fischmann A, Machado PM, Reilly MM, Yousry TA, et al. MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol. 2016;15:65–77.
- Carlier PG, Reyngoudt H. The expanding role of MRI in neuromuscular disorders. Nat Rev Neurol. 2020;16:301–2.
- Müller M, Dohrn MF, Romanzetti S, Gadermayr M, Reetz K, Krämer NA, et al. Semi-automated volumetry of MRI serves as a biomarker in neuromuscular patients. Muscle Nerve. 2020;61:600–7.
- Dahlqvist JR, Widholm P, Leinhard OD, Vissing J. MRI in neuromuscular diseases: an emerging diagnostic tool and biomarker for prognosis and efficacy. Ann Neurol. 2020;88:669–81.
- Czaplinski A, Yen AA, Appel SH. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J Neurol Neurosurg Psychiatry. 2005;77:390–2.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21.
- Zucchi E, Bonetto V, Sorarù G, Martinelli I, Parchi P, Liguori R, et al. Neurofilaments in motor neuron disorders: towards promising diagnostic and prognostic biomarkers. Mol Neurodegener. 2020;15: 58.
- Poesen K, Van Damme P. Diagnostic and prognostic performance of neurofilaments in ALS. Front Neurol. 2019;9:1–7.
- Majumder V, Gregory JM, Barria MA, Green A, Pal S. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis. BMC Neurol. 2018;18:1–7.
- Verber NS, Shepheard SR, Sassani M, McDonough HE, Moore SA, Alix JJP, et al. Biomarkers in motor neuron disease: a state of the art review. Front Neurol. 2019;10:1–28.
- Neuwirth C, Nandedkar S, Stålberg E, Weber M. Motor Unit Number Index (MUNIX): a novel neurophysiological technique to follow disease progression in amyotrophic lateral sclerosis. Muscle Nerve. 2010;42:379–84.
- Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph Lateral Scler. 2012;13:439–45.
- NHLBI. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [Internet]. 2014. p. 1–4. Available at: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed February 9, 2021.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Cross sectional study newcastle – Ottawa quality assessment scale. PLoS One 2016;11:1–2.
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119:497–503.
- Cha CH, Patten BM. Amyotrophic lateral sclerosis: abnormalities of the tongue on magnetic resonance imaging. Ann Neurol. 1989;25:468–72.
- Bryan WW, Reisch JS, McDonald G, Herbelin LL, Barohn RJ, Fleckenstein JL. Magnetic resonance imaging of muscle in amyotrophic lateral sclerosis. Neurology. 1998;51:110–3.
- Hamano T, Mutoh T, Hirayama M, Kawamura Y, Nagata M, Fujiyama J, et al. Muscle MRI findings of X-linked spinal and bulbar muscular atrophy. J Neurol Sci. 2004;222:93–7.
- Kronlage M, Knop KC, Schwarz D, Godel T, Heiland S, Bendszus M, et al. Amyotrophic lateral sclerosis versus multifocal motor neuropathy: utility of MR neurography. Radiology. 2019;292:149–56.
- Hensiek N, Schreiber F, Wimmer T, Kaufmann J, Machts J, Fahlbusch L, et al. Sonographic and 3T-MRI-based evaluation of the tongue in ALS. Neuroimage Clin. 2020;26:102233.
- Lee E, Xing F, Ahn S, Reese TG, Wang R, Green JR, et al. Magnetic resonance imaging based anatomical assessment of tongue impairment due to amyotrophic lateral sclerosis: a preliminary study. J Acoust Soc Am. 2018;143:EL248.
- Jenkins TM, Burness C, Connolly DJ, Rao DG, Hoggard N, Mawson S, et al. A prospective pilot study measuring muscle volumetric change in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:414–23.
- Staff NP, Amrami KK, Howe BM. Magnetic resonance imaging abnormalities of peripheral nerve and muscle are common in amyotrophic lateral sclerosis and share features with multifocal motor neuropathy. Muscle Nerve. 2015;52:137–9.
- Gerevini S, Agosta F, Riva N, Spinelli EG, Pagani E, Caliendo G, et al. MR imaging of brachial plexus and limb-girdle muscles in patients with amyotrophic lateral sclerosis. Radiology. 2016;279:553–61.
- Jenkins TM, Alix JJPP, David C, Pearson E, Rao DG, Hoggard N, et al. Imaging muscle as a potential biomarker of denervation in motor neuron disease. J Neurol Neurosurg Psychiatry. 2018;89:248–55.
- Diamanti L, Alfonsi E, Ferraro OE, Cereda C, Pansarasa O, Bastianello S, et al. A pilot study assessing T1-weighted muscle MRI in amyotrophic lateral sclerosis (ALS). Skeletal Radiol. 2019;48:569–75.
- Klickovic U, Zampedri L, Sinclair CDJJ, Wastling SJ, Trimmel K, Howard RS, et al. Skeletal muscle MRI differentiates SBMA and ALS and correlates with disease severity. Neurology. 2019;93:E895–907.
- Diamanti L, Paoletti M, Vita U, Di Muzic SI, Cereda C, Ballante E, et al. MRI study of paraspinal muscles in patients with amyotrophic lateral sclerosis (ALS). J Clin Med. 2020;9: 934.
- Jenkins TM, Alix JJPP, Fingret J, Esmail T, Hoggard N, Baster K, et al. Longitudinal multi-modal muscle-based biomarker assessment in motor neuron disease. J Neurol. 2020;267:244–56.
- Sproule DM, Punyanitya M, Shen W, Dashnaw S, Martens B, Montgomery M, et al. Muscle volume estimation by magnetic resonance imaging in spinal muscular atrophy. J Child Neurol. 2011;26:309–17.
- Sproule DM, Montgomery MJ, Punyanitya M, Shen W, Dashnaw S, Montes J, et al. Thigh muscle volume measured by magnetic resonance imaging is stable over a 6-month interval in spinal muscular atrophy. J Child Neurol. 2011;26:1252–9.
- Souza PVS, Pinto WBVR, Ricarte A, Badia BML, Seneor DD, Teixeira DT, et al. Clinical and radiological profile of patients with spinal muscular atrophy type 4. Eur J Neurol. 2021;28:609–19.
- Bonati U, Holiga Š, Hellbach N, Risterucci C, Bergauer T, Tang W, et al. Longitudinal characterization of biomarkers for spinal muscular atrophy. Ann Clin Transl Neurol. 2017;4:292–304.
- Mercuri E, Messina S, Kinali M, Cini C, Longman C, Battini R, et al. Congenital form of spinal muscular atrophy predominantly affecting the lower limbs: a clinical and muscle MRI study. Neuromuscul Disord. 2004;14:125–9.
- Durmus H, Yilmaz R, Gulsen-Parman Y, Oflazer-Serdaroglu P, Cuttini M, Dursun MM. u, et al. Muscle magnetic resonance imaging in spinal muscular atrophy type 3: selective and progressive involvement. Muscle Nerve. 2017;55:651–6.
- Kollmer J, Hilgenfeld T, Ziegler A, Saffari A, Sam G, Hayes JM, et al. Quantitative MR neurography biomarkers in 5q-linked spinal muscular atrophy. Neurology. 2019;93:e653–e664.
- Barp A, Carraro E, Albamonte E, Salmin F, Lunetta C, Comi GP, et al. Muscle MRI in two SMA patients on nusinersen treatment: a two years follow-up. J Neurol Sci. 2020;417:117067.
- Brogna C, Cristiano L, Verdolotti T, Pichiecchio A, Cinnante C, Sansone V, et al. MRI patterns of muscle involvement in type 2 and 3 spinal muscular atrophy patients. J Neurol. 2020;267:898–912.
- Liu GC, Jong YJ, Chiang CH, Yang CW. Spinal muscular atrophy: MR evaluation. Pediatr Radiol. 1992;22:584–6.
- Otto LAM, van der Pol WL, Schlaffke L, Wijngaarde CA, Stam M, Wadman RI, et al. Quantitative MRI of skeletal muscle in a cross-sectional cohort of patients with spinal muscular atrophy types 2 and 3. NMR Biomed. 2020;33:1–13.
- Otto LAM, Froeling M, van Eijk RPA, Asselman FL, Wadman R, Cuppen I, et al. Quantification of disease progression in spinal muscular atrophy with muscle MRI—a pilot study. NMR Biomed. 2021;34:1–11.
- Savini G, Asteggiano C, Paoletti M, Parravicini S, Pezzotti E, Solazzo F, et al. Pilot study on quantitative cervical cord and muscular MRI in spinal muscular atrophy: promising biomarkers of disease evolution and treatment? Front Neurol. 2021;12:1–18.
- Dahlqvist JR, Oestergaard ST, Poulsen NS, Thomsen C, Vissing J. Refining the spinobulbar muscular atrophy phenotype by quantitative MRI and clinical assessments. Neurology. 2019;92:e548–e559.
- Mercuri E, Talim B, Moghadaszadeh B, Petit N, Brockington M, Counsell S, et al. Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1). Neuromuscul Disord. 2002;12:631–8.
- Gaeta M, Mileto A, Mazzeo A, Minutoli F, Di Leo R, Settineri N, et al. MRI findings, patterns of disease distribution, and muscle fat fraction calculation in five patients with Charcot-Marie-Tooth type 2 F disease. Skeletal Radiol. 2012;41:515–24.
- Fischer D, Kley RA, Strach K, Meyer C, Sommer T, Eger K, et al. Distinct muscle imaging patterns in myofibrillar myopathies. Neurology. 2008;71:758–65.
- Stramare R, Beltrame V, Dal Borgo R, Gallimberti L, Frigo AC, Pegoraro E, et al. La risonanza magnetica nella valutazione delle patologie muscolari: confronto tra distrofia dei cingoli, miopatia a corpi ialini e distrofia miotonica. Radiol Med. 2010;115:585–99.
- Simon NG. Pragmatic approach to muscle MRI biomarkers in motor neuron disease. J Neurol Neurosurg Psychiatry. 2018;89:230.