Abstract
Objective: To identify occupational risk factors for ALS using well-characterized participants with ALS (P-ALS), sibling controls (S-controls), and matched population controls (P-controls) within the National ALS Registry. We also compared oxidative stress (OS) biomarkers between groups. Methods: P-ALS were recruited over 4 years. Demographic, socioeconomic, and medical data were ascertained from medical records and structured interviews. P-ALS were followed prospectively for 2 years or until death, whichever came sooner. S-controls and age-, sex-, race/ethnicity-, and residential location-matched P-controls were recruited over 3 years. Occupational exposure to lead and agricultural chemicals (ACs) were assigned by an occupational hygienist, blinded to case status. OS biomarkers in urine were measured. Results: P-ALS (mean age 62.8 years; 63% males) resided across the United States. Demographic and socioeconomic variables did not differ among P-ALS, S-controls, and P-controls. P-ALS were more likely to report occupations with exposure to lead (adjusted OR (aOR)=2.3, 95% CI 1.1, 4.6) and ACs (aOR = 2.4, 95% CI 1.2, 4.6) compared to pooled controls. Among those with occupations with exposure to both lead and ACs, aOR was 7.2 (95% CI 2.0, 26.1). Urinary 8-oxo-dG was significantly elevated among P-ALS (11.07 ± 5.42 ng/mL) compared to S-controls, P-controls, or pooled controls (pooled 7.43 ± 5.42 ng/mL; p < 0.0001) but was not associated with occupational exposure to either lead or ACs. Conclusions: Findings reveal increased risk of ALS diagnosis among those with occupational exposure to lead and ACs and increased OS biomarkers among cases compared to controls. OS may be an important pathogenic mechanism in ALS.
Keywords:
Introduction
Although there has been moderate progress on the therapeutic front in ALS over the past several years (Citation1,Citation2), the etiology of ALS remains largely unknown. While up to 10% of ALS cases are considered familial ALS (fALS) and many genetic mutations have been discovered, more than half of fALS cases have unknown mutations (Citation3). The remaining 90% of ALS cases, sporadic ALS, represent a complex disease hypothesized to be caused by gene-environment interactions (Citation4–6).
Previous investigations find increases in the odds of exposure to lead and agricultural chemicals (ACs) among ALS cases compared to controls (Citation7–12). A recent meta-analysis confirmed these findings, reporting risk ratios for lead exposure in the range of 1.07–1.90, and for ACs, 0.70–4.70 (Citation13). Electromagnetic field (EMF) is considered another common risk factor in ALS, although its relationship to ALS has been inconsistent (Citation11,Citation14–16). A recent large European study found positive associations between electric shock and low-frequency magnetic field exposure and ALS (Citation17).
Environmental and occupational risk factors may increase overall oxidative stress (OS), hypothesized to be a factor in the pathway leading to ALS (Citation18). Many different environmental risk factors trigger OS inside and outside of the central nervous system (Citation18). In vitro and in vivo studies have shown that lead alone or in combination with other biochemical stressors results in oxidative injury (Citation19–23). Most agricultural organophosphates, along with other ACs, are clearly linked to OS (Citation24–28). Increases in OS biomarkers have been extensively reported in ALS (Citation18,Citation29–32). Repeated exposures to these agents could induce a process that results in epigenetic modifications that accumulate to enhance the degeneration of motor neurons (Citation33). The potential relationship between hazardous occupational exposures and OS in ALS is not yet understood.
To investigate underlying mechanisms of ALS, large studies that include careful assessment of environmental exposures are needed (Citation34). The CDC National ALS Registry (Registry), launched in October 2010, is the largest ALS registry in the USA (Citation35). The Registry is a valuable resource for studies of ALS (Citation36), particularly for environmental studies. Using the Registry, we enrolled participants with ALS (P-ALS) from across the USA. We conducted a case-control study with two control groups: sibling controls (S-controls) and population-based controls (P-controls). Data collection methods were largely based on our previous study of 355 P-ALS, the ALS Cohort Study of Multicenter Oxidative Stress (ALS COSMOS) (Citation37), one of the studies that best characterized the natural history of ALS and included the establishment of telephone-based data ascertainment methods (Citation38–41). Here, we extend upon ALS COSMOS by considering occupational risk factors for ALS onset. We hypothesize that exposure to occupational factors which increase OS (notably lead and ACs) will increase risk of ALS onset compared to controls (Citation18).
Methods
Study approval
The study protocol was approved by the Columbia University IRB, and all participants gave written informed consent. Research Triangle Institute International (RTI) (tasked to identify population controls [P-controls]) received separate IRB approval. CDC IRB approval was not required as the research was supervised by Columbia University and was investigator-initiated.
P-ALS eligibility criteria
All members of the Registry (∼9000) were considered eligible and invited to participate in this study. Once a participant indicated interest, we applied our a priori inclusion and exclusion criteria used in ALS COSMOS (Citation37) that included proficiency in English.
Publicizing the study project
ARREST ALS (ATSDR Risk Epidemiologic Study of ALS) recruitment relied on potential participants’ interest and willingness to volunteer. Multiple study flyers were sent to US ALS clinics and disease organizations. Additionally, the ATSDR disseminated a brochure to Registry enrollees every three months throughout the study period.
Confirming ALS diagnosis
Upon obtaining medical records, the research team (HM) carefully evaluated neurological examinations, including any necessary ancillary tests, to confirm the diagnosis of ALS. Because recruited P-ALS were diagnosed at major ALS centers, all diagnoses were confirmed (Citation42).
Caregiver involvement
Caregivers provided informed consent to participate and assisted in the study when P-ALS were unable to speak due to dysarthria.
Control participants
To recruit siblings (S-controls), we asked each P-ALS about their family composition and selected the same-sex sibling closest in age to the P-ALS. In the event that this sibling could not participate, we chose another sibling who was also close in age. Siblings were included to control for early life factors (Citation43,Citation44).
The second group of controls was population-based (matched on age ±5 years, sex, race/ethnicity, and residential area) (P-controls). We contracted with RTI International (Research Triangle Park, NC, USA) to identify eligible potential controls. They used geographic Federal Information Processing Standards (FIPS) (the five-digit numbers which uniquely identify geographic area: the first two digits identify state-level and the last three digits identify county-level areas) to find geographic-area matched controls. During the study, age matching was relaxed to ±10 years, sex matching was removed, and FIPS matching was relaxed in order to increase the number of eligible controls who indicated that they would participate. P-controls were required to be fluent in English. RTI only identified potential control candidates who agreed to be interviewed by the Columbia University research staff and did not participate in obtaining study data.
Occupational risk factors
We used a structured interview (designed to minimize recall bias for occupational exposures) to query basic information on occupational and military history with additional questions designed to identify occupations with exposure to lead, EMFs, and ACs. ACs comprised 180 potential neurotoxicants, including most common pesticides, herbicides, and insecticides. The interview was developed by an experienced occupational hygienist (Citation45) and used previously in studies of essential tremor (Citation37,Citation46). describes the content of the structured interview. The occupational hygienist, blinded to case/control status, coded all data for potential exposures () (Citation45).
Table 1 Environmental exposure assessment.
Table 2 Blinded analyses of exposure levels to lead and AC based on structured interviewsa.
Structured interview
Interviewers trained by senior investigators administered structured interviews over the telephone for all participants, as in ALS COSMOS (Citation37). Three hours were required for the baseline interview, which was carefully paced to prevent P-ALS’ fatigue. After each interview, data were entered into a web-based database system.
Survival
Death was defined as when the P-ALS died of any cause, received a tracheostomy, or required chronic assisted ventilation for 23 h daily for 14 consecutive days. Survival status for all enrolled P-ALS was ascertained with a closing date of 15 June 2020.
Biosample collection
For all cases and controls, overnight fasting urine samples were collected by participants at home and shipped overnight in chilled packages to the EHS Laboratory at Columbia University (RMS) (Citation37). Urinary OS biomarkers, isoprostane, and 8-oxodeoxyguanosine (8-oxo-dG) were measured using ELISA. The isoprostane ELISA was well correlated with a GC/MS method of analysis (Oxford Biomedical, Oxford, UK). We previously demonstrated a correlation between 8-oxo-dG ELISA and HPLC using electrochemical detection (Citation30).
Statistical analyses
We compared distributions of continuous variables using means when normally distributed and medians when skewed. Two-sample t-tests were used to compare means of normally distributed continuous variables. Chi-squared tests were used to evaluate associations between categorical variables; Fisher’s exact test was implemented when cell numbers were sparse (<5). Spearman correlations were used to assess the correlation between OS biomarkers. An alpha level of 0.05 was used for all significance testing. Data were analyzed using all available cases, and missing data were minimal (noted in tables).
To evaluate the associations between OS biomarkers and occupational exposures with case status, we used multivariable logistic regression models, adjusted for age. We excluded occupational exposures in the most recent five years to approximate a latency period between exposure and disease onset. As a sensitivity analysis, we varied the latency period using 3, 7, and 10 years and then re-ran our logistic regression models. We also dichotomized occupational exposure duration at the median to create “long” vs. “short” duration categories (3.0 years (lead) and 3.7 years (ACs)).
We first performed all statistical analyses keeping S-controls and P-controls separate. After observing no meaningful differences in occupational exposures between S-controls and P-controls, we combined the two control types to increase power. Due to the low frequency of occupational exposures, we limited covariate adjustment, although we did evaluate sex, age, and race as potential confounders. Regarding age, there were only 6 P-ALS, 2 S-controls, and 2 P-controls under age 50; we included them in the primary analysis but dichotomized age at 65 to account for a possible skewed distribution. In a secondary analysis, we excluded those under age 50 and used age as a continuous variable.
We accounted for matching by adjusting for matched variables in logistic regression models for our primary analysis (Citation48). Due to the limited sample size, we adjusted for age and sex in separate models and compared results, rather than adjusting for both in the same model. We did not adjust for race/ethnicity due to the extremely low prevalence of nonwhite individuals in the sample (). In a sensitivity analysis, we compared conditional logistic regression models to account for matching by age, sex, race/ethnicity, and geographic area in cases with P-controls to unconditional models in the same group, adjusting separately for age and sex (Supplemental Table 1).
Table 3 Sociodemographic characteristics of P-ALS and S-controls and P-controls controls.
To evaluate if lead, AC exposure, and OS biomarkers were associated with time to death in cases, we used Cox proportional hazards models, adjusted for binary age (and months between symptom onset and baseline for OS biomarkers). Person-time was calculated from the time of symptom onset until death, loss to follow up, or study end. The proportional hazards assumption was assessed by evaluating the interaction between exposure and the natural log of person-time.
Results
Participants with ALS (P-ALS)
A total of 227 potential candidates were screened; 103 (45.4%) were enrolled over a 46-month period. Among those not enrolled, 54 had symptoms for a longer duration than the enrollment criteria (initially <18 months); 15 had fALS; 18 could not be recontacted; 17 declined; seven died or received tracheostomy; and 13 were ineligible for other reasons. Because of slow enrollment in the first two years, we relaxed criteria to include symptom onset in the past 24 months (46 participants were already enrolled when this change was made). The US geographic distribution was wide for both recruited ALS participants and controls (). Disease characteristics of enrolled P-ALS are shown in .
Figure 1 Geographic distribution of P-ALS (participants with ALS), S-controls (siblings), and P-controls (population). P-ALS are widely distributed throughout the USA, as are S-controls and P-controls.
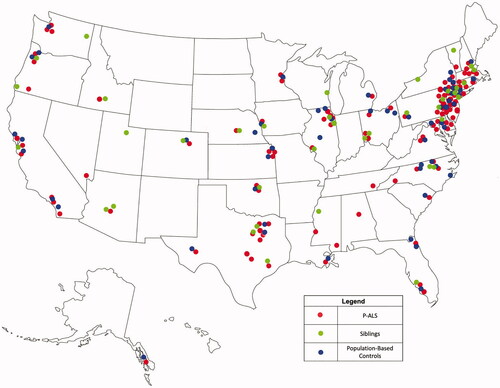
Table 4 Baseline disease characteristics of participants with ALS (N = 103).
Controls
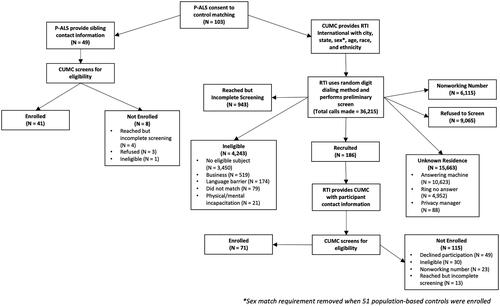
We recruited 40 S-controls. Less than half of P-ALS had available siblings, and the majority were of the opposite sex (). For P-controls, RTI International identified 186 potential candidates, representing 0.5% of all recruitment telephone calls. Details of control recruitment are shown in .
Figure 2 Recruitment and enrollment of P-ALS, S-controls, and P-controls are shown in a flow chart. All the participants (P-ALS, S-controls, and P-controls) received identical structured interviews.
Sociodemographic characteristics are summarized in .
Occupational risk factors
Compared to S-controls and P-controls, P-ALS were more frequently classified as having possible or probable occupational exposure to lead and ACs (). Although EMF exposures were included as part of the study goals, exposure to EMF was extremely low in all groups to make meaningful comparisons. Because sociodemographic features did not differ between S-controls and P-controls, we combined them in further analyses.
Table 5 Occupational exposure to lead, electromagnetic fields, and agricultural chemicals.
Adjusting for age, P-ALS had approximately two times the odds of being exposed to lead compared to pooled controls and two times the odds of being exposed to ACs compared to controls (). Results were consistent when we adjusted for age, sex, or accounted for P-control matching using conditional logistic regression models (, Supplemental Table 1), indicating robustness in methods used to adjust for matching.
Table 6 Logistic regression models for associations between occupational exposures and case/control status.
Four times as many P-ALS (12%) experienced exposure to both lead and ACs than controls (3%) (). Compared to controls, P-ALS had approximately seven times the odds of being exposed to both lead and ACs (). While there were no significant differences in median years of exposure comparing cases and controls (data not shown), longer duration of lead exposure was significantly associated with ALS, controlling for age. For ACs, aORs for long and short duration of exposure were similar (). When lead and ACs were included in the same model, the resulting odds ratios were of similar magnitude to those found in models which evaluated exposures separately. Exposure to lead and ACs were only slightly correlated (Spearman r = 0.2, p < 0.01), indicating that bias amplification should not be expected in these models (Citation49). When we varied the length of the latency period used in a sensitivity analysis, resulting ORs were nearly identical (Supplemental Table 2).
Table 7 Extent of overlap in occupational exposures for cases and pooled controls.
Biomarker results
Urinary concentrations of 8-oxodG and isoprostane were significantly positively correlated (r = 0.30, p < 0.0001). Urinary 8-oxodG in P-ALS was significantly higher than in controls () but isoprostane concentrations were not different. In logistic regression models combining control types and adjusting for binary age, the odds ratios for isoprostane and 8-oxodG were 1.2 (0.9, 1.7) (p = 0.28) and OR 1.2 (1.1, 1.3) for 8-oxo-dG (p < 0.0001), respectively, per one unit increase in concentration. There were no significant correlations between OS biomarker values and duration of occupational exposure to lead (isoprostane: r = 0.05, p = 0.75, 8-oxo-dG: r = 0.14, p = 0.39) or ACs (isoprostane: r= −0.05, p = 0.75, 8-oxo-dG: r= −0.17, p = 0.23).
Table 8 Comparison of oxidative stress (OS) biomarkers levels in cases and controls.
Survival data
As of June 2020, survival status was available for 72 of 103 P-ALS, all others were censored. There was a wide range of survival duration; the mean total survival (from symptom onset to death) was 38.0 months (SD 16.8) with a minimum of 9.0 and maximum of 94.3 months, within previously reported ranges (Citation50). Ever-lead and ever-AC exposure were not significantly associated with time to death in age-adjusted models (). OS biomarker 8-oxo-dG may be related to survival (HR 1.03 (0.99, 1.08)), while isoprostane was not appreciably associated (HR 1.06 (0.80, 1.39)) when adjusted for age and months between symptom onset and baseline, consistent with previous findings (Citation51).
Table 9 Cox proportional hazards models for associations between occupational exposures and time to death in cases (n = 94).
Sensitivity analyses
Excluding the few cases and controls under age 50 years and using age as a continuous covariate did not substantially change the associations of interest nor did it change the interpretation of the results (Supplemental Tables 3 and 4).
Discussion
Our results suggest that ALS patients were more likely to have experienced occupational exposure to lead and/or ACs compared to controls, confirming the work of previous studies. We found that longer duration of lead exposure may have a dose-response association with ALS, whereas any duration of exposure to ACs conferred higher odds of ALS.
Detailed assessment of occupational exposures is a strength of our study. The structured interview was designed by a board-certified industrial hygienist, who assigned exposures blinded to case-control status. In a study of essential tremor using this instrument, higher blood lead concentrations were found in individuals who were identified as having possible or probable occupational exposure to lead (Citation46), which confers increased confidence in the utility of this tool. We observed a significant difference in exposure to lead among P-ALS compared to siblings that were particularly striking, and P-ALS also had significantly increased exposure to ACs as compared to S-controls and P-controls.
Also striking was that independently, associations between lead and AC exposures and ALS were modest; however, exposure to both lead and ACs resulted an aOR that appeared supermultiplicative. This suggests the possibility of synergistic neurotoxic effects, but we are cautious in interpretation due to the relatively small number of individuals exposed to both lead and ACs. Nonetheless, this finding has support from experimental models. Concurrent exposure to exogenous glutamate and lead increases neuronal cell death via mechanisms that increase OS and decrease intracellular glutathione defenses against OS (Citation52). In another model, spinal cord and dorsal root ganglion cells obtained from E12 mouse embryos were exposed in culture to low levels of the ACs paraquat, glutamate, and thermal stress, individually and in combination (Citation53). Paraquat administered alone was associated with cell death in a dose-dependent manner, and this effect was multiplied when paraquat-treated cultures were subjected to heat shock (Citation53). This model is particularly intriguing as the combination of low-level oxidative stressors was associated with motor neuron vulnerability to environmental stressors and may in part explain the mid- to late-life symptom onset among both familial and sporadic ALS (Citation53). In vitro studies attempt to mimic the human environment in which multiple stressors are likely involved, resulting in interactions (Citation54,Citation55).
We have hypothesized that there may not be a single environmental risk factor associated with ALS. Rather, diverse risk factors that adversely affect individuals susceptible to different environmental neurotoxicants are more likely (Citation18). A final common path to neuronal damage may be OS. Our finding that the urinary OS biomarker, 8-oxo-dG (reflecting oxidized DNA), was elevated among P-ALS compared to siblings and P-controls was striking. Isoprostane (a lipid peroxidase product) level, although not statistically different between cases and controls, was highly correlated with 8-oxo-dG, supporting some involvement of both OS biomarkers. Because OS biomarker measurements only reflect recent OS status, we cannot conclude that abnormal OS markers were the result of previous environmental exposures; indeed, OS biomarkers did not differ among those with or without exposure to lead and ACs (data not shown). To better elucidate this relationship, studies will need to acquire biosamples to measure OS more proximally to when occupational exposures have occurred. Increased OS has direct implications for treatment, as demonstrated in past studies of vitamin E (Citation56,Citation57), CoQ10 (Citation58), and Edaravone, the second approved medication for the treatment of ALS (Citation1). Edaravone treatment reduced 3-nitrotyrosine (3NT), a nitrosative stress biomarker, in a previous study (Citation32).
The residential geographic range of recruited P-ALS and controls broadly reflects the USA, likely representing a microcosm of the Registry distribution in this country (Citation36). Still, our case-control study has several limitations, mainly related to our sample size and characteristics. First, the Registry relies on voluntary participation; participation in ancillary studies, particularly observational studies, like this one requires a second level of volunteerism. Thus, generalizability to the entire ALS population is likely limited, and our results should only be generalized to those cases who would agree to participate in such research. Nonetheless, our study sample did not differ in many disease characteristics when compared to ALS COSMOS (Citation37–41,Citation59). In ALS COSMOS, P-ALS were recruited from multiple ALS clinics, and no differences were found between enrolled P-ALS and those eligible but not enrolled (Citation37). Thus, we assert that the ARREST P-ALS were similar to the ALS COSMOS cohort and likely represent P-ALS seen at ALS Clinics (Citation37).
Second, we had a relatively small number of P-ALS and controls and could therefore not adjust for many covariates in our analyses. Interpretation of our regression models must be done cautiously. Larger studies have recently been completed that improve on these limitations by utilizing a pooled case-control study design which achieved a much larger sample size (Citation17). These investigators were therefore able to investigate the relationship between EMFs and ALS, an exposure which we could not study here due to very small numbers of highly exposed individuals (Citation17). We aim to investigate exposure to EMFs in future pooled analyses of ARREST and COSMOS studies in order to address this limitation. This will be important, as previous studies have found relationships between white collar jobs and increased risk of death due to ALS (Citation60). Therefore, further understanding of how occupations relate to ALS is needed.
Third, the telephone-based recruitment methods were time-consuming, which may have deterred participation. Fourth, only one industrial hygienist coded the exposures, representing both a strength and weakness; a strength for consistency in ratings and a weakness, as a panel may have minimized the potential for systematic misclassification. However, resource limitations precluded more than one rater. Nevertheless, we identified environmental risk factors and demonstrated that conducting a successful case-control study nested within an ALS disease registry is possible.
Based on our study and other reports, we stress the need for use of protective equipment and other measures by those who work in occupations associated with exposure to lead, ACs (pesticides, insecticides, and herbicides), and especially multiple neurotoxicants. In summary, we designed a case-control study using P-ALS enrolled in the CDC National ALS Registry along with S-controls and P-controls. We found evidence that lead and ACs may be significant risk factors for ALS, possibly causing OS. In support of our hypothesis, we found elevated urinary OS biomarkers in P-ALS (Citation18).
Author contributions
All authors contributed to the manuscript, including assisting in the planning stages, grant preparation, data collection, data analyses, interpretation, writing the draft, and approving the final manuscript.
Supplemental Material
Download MS Word (41.1 KB)Acknowledgments
We are grateful for the study P-ALS and their families and siblings, as well as the control participants for their time and interest in the study. We are very appreciative of the encouragement and strong support extended to our research by the ATSDR/CDC study team, Judith Smith, PhD, Wendy Kaye, PhD, and other colleagues. The CDC Contract (200-2013-568886 and R01TS000243 to HM) and MDA Wings Over Wall Street fund supported this project. We acknowledge the late Ray Goetz, PhD, Columbia University, who performed the early statistical analyses. RTI International, NC (Thomas Duffy, Senior Director) oversaw the service that identified matched controls. Dr. Daragh Heitzman, Texas Neurology; Dr. Eric Sorenson, Mayo Clinic Rochester; Dr. Björn Oskarsson, Mayo Clinic Jacksonville; and Dr. J Americo M. Fernandes, University of Nebraska assisted with participant recruitment at their ALS Centers. Jessica Singleton, BA, Brittany McHale, LCSW, Camila Ibagon, BS, and Marie-France Likanje, MA assisted with conducting structured interviews and data entry. Irina Gurvich, MS prepared the biosamples in the Biomarkers Laboratory supported by ES009089, and Iryna Sirosh MS and Qiao Wang analyzed urinary oxidative stress markers. Saliva DNA was prepared by the Genome Center, Department of Neurology, and C9crf72 repeats (only in ALS participants) were processed and analyzed by Ali Naini, PhD, Pathology, Columbia University. Georgia Christodoulou, MA, University of Southern California, assisted in editing the manuscript. Cassandra Telerico, PhD, Cleveland Clinic, edited the final manuscript.
Disclosure statement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of ATSDR, CDC, and/or the Department of Health and Human Services (HHS). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Writing G, Edaravone A. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:505–12.
- Paganoni S, Macklin EA, Hendrix S, Berry JD, Elliott MA, Maiser S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383:919–30.
- Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim Biophys Acta. 2015;1852:679–84.
- van den Berg LH, Sorenson E, Gronseth G, Macklin EA, Andrews J, Baloh RH, et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology. 2019;92:e1610–e23.
- Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–28.
- Riancho J, Bosque-Varela P, Perez-Pereda S, Povedano M, de Munain AL, Santurtun A. The increasing importance of environmental conditions in amyotrophic lateral sclerosis. Int J Biometeorol. 2018;62:1361–74.
- Kamel F, Umbach DM, Munsat TL, Shefner JM, Hu H, Sandler DP. Lead exposure and amyotrophic lateral sclerosis. Epidemiology. 2002;13:311–9.
- Kamel F, Umbach DM, Hu H, Munsat TL, Shefner JM, Taylor JA, et al. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:195–201.
- Fang F, Peters TL, Beard JD, Umbach DM, Keller J, Mariosa D, et al. Blood lead, bone turnover, and survival in amyotrophic lateral sclerosis. Am J Epidemiol. 2017;186:1057–64.
- Malek AM, Barchowsky A, Bowser R, Heiman-Patterson T, Lacomis D, Rana S, et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neurodegener Dis. 2014;14:31–8.
- Filippini T, Tesauro M, Fiore M, Malagoli C, Consonni M, Violi F, et al. Environmental and occupational risk factors of amyotrophic lateral sclerosis: a population-based case-control study. Int J Environ Res Public Health. 2020;17:2882.
- Meng E, Mao Y, Yao Q, Han X, Li X, Zhang K, et al. Population-based study of environmental/occupational lead exposure and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Neurol Sci. 2020;41:35–40.
- Gunnarsson LG, Bodin L. Amyotrophic lateral sclerosis and occupational exposures: a systematic literature review and meta-analyses. Int J Environ Res Public Health. 2018;15:2371.
- Riancho J, Sanchez de la Torre JR, Paz-Fajardo L, Limia C, Santurtun A, Cifra M, et al. The role of magnetic fields in neurodegenerative diseases. Int J Biometeorol. 2021;65:107–17.
- Luna J, Leleu JP, Preux PM, Corcia P, Couratier P, Marin B, et al. Residential exposure to ultra high frequency electromagnetic fields emitted by Global System for Mobile (GSM) antennas and amyotrophic lateral sclerosis incidence: a geo-epidemiological population-based study. Environ Res. 2019;176:108525.
- Vinceti M, Malagoli C, Fabbi S, Kheifets L, Violi F, Poli M, et al. Magnetic fields exposure from high-voltage power lines and risk of amyotrophic lateral sclerosis in two Italian populations. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:583–9.
- Peters S, Visser AE, D’Ovidio F, Beghi E, Chio A, Logroscino G, et al. Associations of electric shock and extremely low-frequency magnetic field exposure with the risk of amyotrophic lateral sclerosis. Am J Epidemiol. 2019;188:796–805.
- D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–27.
- Chetty CS, Vemuri MC, Campbell K, Suresh C. Lead-induced cell death of human neuroblastoma cells involves GSH deprivation. Cell Mol Biol Lett. 2005;10:413–23.
- Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. The involvement of copper transporter in lead-induced oxidative stress in astroglia. Neurochem Res. 2005;30:429–38.
- Fowler BA, Whittaker MH, Lipsky M, Wang G, Chen XQ. Oxidative stress induced by lead, cadmium and arsenic mixtures: 30-day, 90-day, and 180-day drinking water studies in rats: an overview. Biometals. 2004;17:567–8.
- Prasanthi RP, Devi CB, Basha DC, Reddy NS, Reddy GR. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci. 2010;28:161–7.
- Ahamed M, Siddiqui MK. Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta. 2007;383:57–64.
- Shadnia S, Azizi E, Hosseini R, Khoei S, Fouladdel S, Pajoumand A, et al. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol. 2005;24:439–45.
- Franco R, Sanchez-Olea R, Reyes-Reyes EM, Panayiotidis MI. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat Res. 2009;674:3–22.
- Schmuck G, Rohrdanz E, Tran-Thi QH, Kahl R, Schluter G. Oxidative stress in rat cortical neurons and astrocytes induced by paraquat in vitro. Neurotox Res. 2002;4:1–13.
- Muniz JF, McCauley L, Scherer J, Lasarev M, Koshy M, Kow YW, et al. Biomarkers of oxidative stress and DNA damage in agricultural workers: a pilot study. Toxicol Appl Pharmacol. 2008;227:97–107.
- Mbah Ntepe LJ, Habib R, Judith LN, Raza S, Nepovimova E, Kuca K, et al. Oxidative stress and analysis of selected SNPs of ACHE (rs 2571598), BCHE (rs 3495), CAT (rs 7943316), SIRT1 (rs 10823108), GSTP1 (rs 1695), and Gene GSTM1, GSTT1 in chronic organophosphates exposed groups from Cameroon and Pakistan. Int J Mol Sci. 2020;21:6432.
- Blasco H, Garcon G, Patin F, Veyrat-Durebex C, Boyer J, Devos D, et al. Panel of oxidative stress and inflammatory biomarkers in ALS: a pilot study. Can J Neurol Sci. 2017;44:90–5.
- Mitsumoto H, Santella RM, Liu X, Bogdanov M, Zipprich J, Wu HC, et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph Lateral Scler. 2008;9:177–83.
- Simpson EP, Yen AA, Appel SH. Oxidative stress: a common denominator in the pathogenesis of amyotrophic lateral sclerosis. Curr Opin Rheumatol. 2003;15:730–6.
- Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotroph Lateral Scler. 2006;7:241–5.
- Eisen A, Kiernan M, Mitsumoto H, Swash M. Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry. 2014;85:1232–8.
- Factor-Litvak P, Al-Chalabi A, Ascherio A, Bradley W, Chio A, Garruto R, et al. Current pathways for epidemiological research in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14: 33–43.
- Mehta P, Antao V, Kaye W, Sanchez M, Williamson D, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis – United States, 2010–2011. MMWR Suppl. 2014;63:1–14.
- Mehta P, Kaye W, Raymond J, Punjani R, Larson T, Cohen J, et al. Prevalence of amyotrophic lateral sclerosis – United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67:1285–9.
- Mitsumoto H, Factor-Litvak P, Andrews H, Goetz RR, Andrews L, Rabkin JG, et al. ALS Multicenter Cohort Study of Oxidative Stress (ALS COSMOS): study methodology, recruitment, and baseline demographic and disease characteristics. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:192–203.
- Rabkin JG, Goetz R, Factor-Litvak P, Hupf J, McElhiney M, Singleton J, et al. Depression and wish to die in a multicenter cohort of ALS patients. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:265–73.
- Rabkin J, Goetz R, Murphy JM, Factor-Litvak P, Mitsumoto H, Group ACS. Cognitive impairment, behavioral impairment, depression, and wish to die in an ALS cohort. Neurology. 2016;87:1320–8.
- Nieves JW, Gennings C, Factor-Litvak P, Hupf J, Singleton J, Sharf V, et al. Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol. 2016;73:1425–32.
- Murphy J, Factor-Litvak P, Goetz R, Lomen-Hoerth C, Nagy PL, Hupf J, et al. Cognitive-behavioral screening reveals prevalent impairment in a large multicenter ALS cohort. Neurology. 2016;86:813–20.
- Horton DK, Graham S, Punjani R, Wilt G, Kaye W, Maginnis K, et al. A spatial analysis of amyotrophic lateral sclerosis (ALS) cases in the United States and their proximity to multidisciplinary ALS clinics, 2013. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:126–33.
- Nelson LM, Tanner CM, Van Den Eeden S, McGuire VM, editors. Neuroepidemiology. From principles to practice. New York, NY: Oxford University Press; 2004.
- Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol. 1992;135:1029–41.
- Ann DH. The occupational environment: its evaluation, control, and management. Falls Church, VA: American Industrial Hygiene Association Press; 2014.
- Louis ED, Jurewicz EC, Applegate L, Factor-Litvak P, Parides M, Andrews L, et al. Association between essential tremor and blood lead concentration. Environ Health Perspect. 2003;111:1707–11.
- Bowman JD, Touchstone JA, Yost MG. A population-based job exposure matrix for power-frequency magnetic fields. J Occup Environ Hyg. 2007;4:715–28.
- Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969.
- Weisskopf MG, Seals RM, Webster TF. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ Health Perspect. 2018;126:047003.
- Mitsumoto H, Chad DA, Pioro EP. Amyotrophic lateral sclerosis. New York, NY: Oxford University Press; 1998:18–33.
- Mitsumoto H, Garofalo DC, Santella RM, Sorenson EJ, Oskarsson B, Fernandes JAM, Jr, et al. Plasma creatinine and oxidative stress biomarkers in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:263–72.
- Loikkanen J, Naarala J, Vahakangas KH, Savolainen KM. Glutamate increases toxicity of inorganic lead in GT1-7 neurons: partial protection induced by flunarizine. Arch Toxicol. 2003;77:663–71.
- Kriscenski-Perry E, Durham HD, Sheu SS, Figlewicz DA. Synergistic effects of low level stressors in an oxidative damage model of spinal motor neuron degeneration. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:151–7.
- Sahin E., Gümüşlü S. Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav Brain Res. 2004;155:241–8.
- Quandt SA, Jones BT, Talton JW, Whalley LE, Galvan L, Vallejos QM, et al. Heavy metals exposures among Mexican farmworkers in eastern North Carolina. Environ Res. 2010;110:83–8.
- Desnuelle C, Dib M, Garrel C, Favier A. A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS riluzole-tocopherol Study Group. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:9–18.
- Graf M, Ecker D, Horowski R, Kramer B, Riederer P, Gerlach M, et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J Neural Transm. 2005;112:649–60.
- Kaufmann P, Thompson JL, Levy G, Buchsbaum R, Shefner J, Krivickas LS, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:235–44.
- Hupf JFL, Goetz R, et al. ARREST ALS: an extension of the ALS COSMOS study. Amyotroph Lateral Scler Frontotemporal Degener. 2018;176–7.
- Beard JD, Steege AL, Ju J, Lu J, Luckhaupt SE, Schubauer-Berigan MK. Mortality from amyotrophic lateral sclerosis and Parkinson’s disease among different occupation groups – United States, 1985–2011. MMWR Morb Mortal Wkly Rep. 2017;66:718–22.
