Abstract
The absence of disease modifying treatments for amyotrophic lateral sclerosis (ALS) is in large part a consequence of its complexity and heterogeneity. Deep clinical and biological phenotyping of people living with ALS would assist in the development of effective treatments and target specific biomarkers to monitor disease progression and inform on treatment efficacy. The objective of this paper is to present the Comprehensive Analysis Platform To Understand Remedy and Eliminate ALS (CAPTURE ALS), an open and translational platform for the scientific community currently in development. CAPTURE ALS is a Canadian-based platform designed to include participants’ voices in its development and through execution. Standardized methods will be used to longitudinally characterize ALS patients and healthy controls through deep clinical phenotyping, neuroimaging, neurocognitive and speech assessments, genotyping and multisource biospecimen collection. This effort plugs into complementary Canadian and international initiatives to share common resources. Here, we describe in detail the infrastructure, operating procedures, and long-term vision of CAPTURE ALS to facilitate and accelerate translational ALS research in Canada and beyond.
Introduction
Amyotrophic lateral sclerosis (ALS) is a highly heterogenous disease, with clinical presentation and disease evolution varying significantly between patients (Citation1). This heterogeneity is ‘suspected to contribute to variability in treatment response and may account for the difficulty identifying effective disease modifying treatments for ALS. Deep clinical, genetic, and biological phenotyping of patients should favor the understanding of ALS pathogenesis and the development of effective treatments and biomarkers. To date, these studies have been limited by small sample size, because existing cohorts are small, scattered across institutions and use disparate methodologies. There is a need to establish an open, multi-site, multidisciplinary platform to increase access to high-quality biological materials from large cohorts of clinically well-defined patients. Here, we describe a national Canadian effort trying to address the clinical heterogeneity of ALS with the collection of a comprehensive biological picture of individuals living with ALS.
The foundations of CAPTURE ALS
CAPTURE ALS was developed to operationalize multidisciplinary research expertise and take into account patients’ desire to participate in ALS research. Its three main objectives are: (1) to unravel the heterogeneity of ALS; (2) to build a widely available biorepository; and (3) to promote the participation of ALS patients in research.
The platform was inspired by and leverages the expertise, infrastructure and clinical investigative model of the Canadian ALS Neuroimaging Consortium (CALSNIC) (Citation2). CALSNIC enables multi-center, prospective, longitudinal imaging-centric studies in ALS (Citation3–8). Due to successful scaling, the original CALSNIC-1 project was expanded through additional funding to increase sample size and test new hypotheses in CALSNIC-2 which is now established at seven Canadian and two American ALS clinics (Citation2).
CAPTURE ALS has partnered with the Clinical Biological Imaging and Genetic Repository (C-BIG) at the Montreal Neurological Institute to serve as its biobank. C-BIG provides the infrastructure and governance to meet CAPTURE ALS’ open science biobanking needs and to ensure its sustainability (Citation9,Citation10). Furthermore, C-BIG currently houses a complementary collection of over 32,500 biosamples from more than 80 disease cohorts. Since November 2020, C-BIG data is gradually being made an Open Science resource to the research community, with ALS, Parkinson’s Disease and healthy control cohorts now openly available (https://cbigr-open.loris.ca/).
CAPTURE ALS protocol
Deep clinical, imaging and biospecimen phenotyping of ALS patients and healthy controls
Participant recruitment and engagement strategy
To help understand the clinical heterogeneity of ALS, patients will be recruited across the ALS spectrum including progressive muscular atrophy (PMA), primary lateral sclerosis (PLS), ALS-FTD, and asymptomatic individuals with known pathogenic ALS-related mutations, hereinafter referred to as “patients”. Diagnosis will be re-confirmed at every visit by a neurologist and if a participant is misdiagnosed as having ALS, their data will be re-coded as “disease control” and excluded from core analyses. Demographically matched healthy controls, including spouses, family members, or friends of the patient, or staff members or their connections, will also be recruited.
CAPTURE ALS will initially launch at four Canadian ALS research centers (University of Toronto, McGill University, University of Alberta and Université Laval). These centers were selected because they have existing research infrastructure and are the top recruiting sites for CALSNIC, reaching approximately 45% of the Canadian ALS patient community. Patients who are not followed in these clinics may participate if they are able to travel to the closest study center. At the time of writing (December 2021), participant enrollment has not yet begun. Phase 1 of the project includes recruitment of 100 patients and 25 controls which is expected to occur from March 2022 to June 2024.
CAPTURE ALS is a patient-centered research initiative, which is greatly dependent on longitudinal data collected from each participant. Thus, active and meaningful participant engagement has been included in the conception of the project and the operational stages. Specifically, ALS patients and caregivers participating in the ALS Talk Project, a Canadian focus group study, affirmed data sharing via Open Science, including with pharmaceutical companies. A Participant Partner Advisory Council (PPAC) has been created, composed of two people affected by ALS from each participating center, which will ensure that the perspective and priorities of people living with ALS and their supporting communities are reflected in CAPTURE ALS. CAPTURE ALS will periodically host participant engagement activities including educational information sessions and study progress updates.
Visit procedures and deep clinical phenotyping
There are four study visits, at 0, 4, 8 and 12 months, for patients and asymptomatic mutation carriers who will undergo the same assessments and two visits, at 0 and 8 months, for healthy controls. The visit schedule is modeled on CALSNIC, from which its clinical and imaging protocols were derived (Citation2). Detailed demographic and medical history and clinical trial involvement will be documented for all participants. At each visit, patients will undergo a neurological exam, complete Edinburgh Cognitive and Behavioral ALS Screen (ECAS) to measure cognition (Citation11,Citation12), and have disability assessed with the revised ALS Functional Rating Scale (ALSFRS-R)(Citation13). A full cognitive panel with evaluation of executive functions, attention, language, memory and social cognition is included at the 0- and 8-month visits for all participants (). Patients will be followed to monitor disease progression through medical record review every 6 months, starting at 18months, until death or study withdrawal.
Figure 1 CAPTURE ALS visit procedures and biosampling protocols. There are four study visits at 0, 4, 8, and 12 months for ALS patients and two visits at 0 and 8 months for healthy controls. At each visit a complete neurological exam, ALSFRS-R disability score, speech analysis and multimodal neuroimaging are performed. The biofluids are collected at every visit, except CSF (which is collected when patients consent to this optional procedure). A full cognitive panel is included at the 0- and 8-month visits. Data are stored in CAPTURE databases and biofluids are sent to C-BIG for open science.
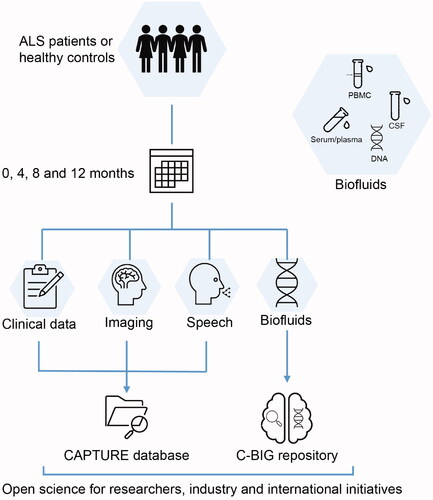
Biospecimens collection
Blood (serum and plasma) is collected at each visit for all participants. Cerebrospinal fluid (CSF) is longitudinally collected for patients who consent to this optional procedure. Standard operating procedures (SOPs) align with those of C-BIG, with attention to harmonization with other similar international initiatives. Fluids are processed locally and shipped for storage at C-BIG. Researchers will have access to collected peripheral blood mononuclear cells (PBMCs) from patients to derive iPSCs. Whole genome sequencing (WGS) and DNA methylation analysis will be conducted on every patient and RNA will be collected from all participants.
Multimodal neuroimaging
CAPTURE ALS leverages the expertise of CALSNIC (Citation3,Citation4,Citation14,Citation15) whose database contains >800 longitudinal MRI scans from more than 200 ALS patients and 150 controls. The imaging protocol, performed at every participant visit, includes T1-weighted images, diffusion tensor imaging (DTI), resting-state functional MRI (fMRI), and MR spectroscopy.
Speech analysis
The speech assessment tasks, performed by all participants within one week of clinical tests, are recorded using an in-house developed online recording platform and analyzed off-line using well established Praat-Parselmouth (Citation16). The measures obtained include speaking and articulatory rates, phonatory (spectral and cepstral) as well as spectral and temporal segmental and suprasegmental characteristics, assessing respiratory, phonatory, articulatory and resonatory speech subsystems (Citation17,Citation18). These assessments will detect early speech difficulty, evaluate the rate of progression in bulbar ALS, and differentiate speech patterns in ALS-FTD and bulbar ALS (Citation19–21).
Open data and sample repository
Ethics and participants’ anonymity
Participation is fully voluntary. Recruitment and data collection respect the Canadian privacy and health information laws and the Tri-Council Policy Statement on Ethical Conduct for Research Involving Humans (Citation22). Participants are assigned a global unique identifier according to NIH-NDA standards (Citation23). Participants provide informed consent permitting storage and sharing of de-identified biosamples and data for future research on ALS, related disorders, and other human diseases by academic and industry researchers.
Cyberinfrastructure and accessibility for researchers, industry and international initiatives
The Longitudinal Online Research and Imaging System (LORIS), used by various national and international projects (Citation2,Citation9,Citation24–28) including CAPTURE ALS, is a web-based database for storage, management, quality control, validation and distribution of multi-center, multimodal data. LORIS’ LIMS (Laboratory Information Management Systems) capabilities handle C-BIG’s biospecimen data (Citation10). LORIS includes numerous quality control and validation checks for each modality of data (Citation29). Having a single database enables the easy integration of data and biosamples required to conduct the rich multimodal patient characterization at the core of CAPTURE ALS. In that regard, we will apply advanced analytical approaches to the multimodal data to establish novel longitudinal markers and predictors of ALS progression and implement a biologically-defined staging and stratification of ALS patients.
The data and materials collected by CAPTURE ALS are made as open and accessible as possible while protecting participant privacy. Based on the potential risk of re-identification, each variable within LORIS is individually assigned an access level (open, registered, controlled) that will dictate the level of oversight required prior to sharing (Citation10). Qualified researchers from academia or industry, including funders of CAPTURE ALS, complete an electronic request for data or samples. Every request is reviewed by the C-BIG Tissue and Data Committee (TDC) and CAPTURE ALS Biosample and Data Sharing Committee (BDSC). Access will depend on the type of data/material being requested and will range from openly sharing data to requiring REB project approval. A standard material and data transfer agreement will be signed to streamline sharing. Data and samples will be re-coded before being shared, and researchers will only receive access to data relevant to their scientific inquiry.
The collaboration of CAPTURE ALS with worldwide platforms and databases
CAPTURE ALS is actively building partnerships with multiple complementary international consortia and welcomes new opportunities for early collaboration, as well as feedback on how to improve the platform.
The Northeast Amyotrophic Lateral Sclerosis consortium (NEALS) provides expertise for clinical trial conduct and manages biobanking for important American initiatives including Answer ALS and Target ALS. CAPTURE ALS will work with NEALS toward harmonization of biosample collection and processing procedures wherever possible. Through existing data transfer agreements and data protection protocols, CAPTURE ALS will help Canada reach the goal of delivering 1000 WGS and associated clinical datasets to Project MinE, an international collaboration out of the Netherlands that aims to identify rare ALS variants through the sequencing of 15,000 patients (Citation30). CAPTURE ALS will also contribute imaging data to the Neuroimaging Society in Amyotrophic Lateral Sclerosis (NiSALS), a central repository containing over 1000 de-identified MRI scans from ALS patients collected at European, American and Australian Centers (Citation31–33).
Within Canada, CAPTURE ALS is aligning its protocol with the Ontario Neurodegenerative Disease Research Initiative (ONDRI) to facilitate co-recruitment of participants. ONDRI collates multidimensional data from patients with Alzheimer’s disease, frontotemporal lobar degeneration, ALS, Parkinson’s disease and cerebrovascular disease (Citation34–37).
Development of CAPTURE ALS
The launch of CAPTURE ALS was made possible by an initial ALS Canada investment which was leveraged into a funded grant partnership between ALS Canada, Brain Canada Regeneron Pharmaceuticals and Alnylam Pharmaceuticals. Industry partners have a seat on the Scientific Committee. This funding allows for key aspects of the first phase of the platform infrastructure to be established and operationalized at the four centers, while planning for subsequent phases. Phase 2 foresees an increase in the platform scope, adding postmortem tissue collection, electrodiagnostics, wearables, and Positron Emission Tomography, and aims to provide a majority of ALS patients in Canada the opportunity to participate in research (). Ultimately, this will require additional research sites.
Figure 2 CAPTURE ALS phase development timeline. CAPTURE ALS is developed in two phases which are interconnected and overlap. Phase 1 includes the development of SOPs, study protocol documents and consent forms, submission of protocols to local research ethics boards, contract negotiations, database building, and creation of a patient-facing website. In phase 2, the platform size and scope are expanded. Additional desirable goals are to incorporate a collection of postmortem brain and spinal cord tissues, electrodiagnostic and wearable-derived data, Positron Emission Tomography, and to extend participant follow-up beyond one year. Some items in phases 2 may begin earlier than expected. Both phases are developed through ongoing collaboration with key stakeholders including patients, the national and international scientific community and industry. These phases are guided toward the goals of unraveling the heterogeneity of ALS, building a broadly available biorepository, and favoring the participation of every ALS patient in research.
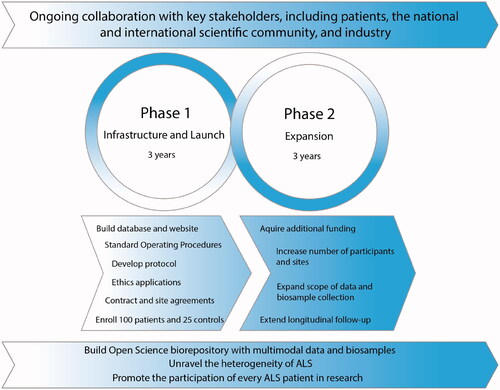
Increasing the scope and size requires new and long-term funding which will be pursued through granting agencies, industry and philanthropy. Because we have adopted a scalable platform architecture, additional funds enable the immediate expansion. Additional funding has already been obtained from the Canadian Institutes of Health Research (CIHR) and a philanthropic donation to increase the number of participants and add RNA sequencing.
Conclusion
CAPTURE ALS is a translational scientific platform, designed for participant engagement through ongoing collaboration with people affected by ALS. CAPTURE ALS will establish a biorepository containing multimodal clinical, cognitive, imaging, speech data and biosamples, which collectively provide a comprehensive resource about people living with ALS. CAPTURE ALS will increase research capacity across Canada and provide an invaluable opportunity for Canadians to participate in global ALS research. Through an open science approach, supported by legal, ethical, and technological infrastructures, these resources will be easily shared worldwide with academics, clinicians, industry, and other consortia. We anticipate that these materials will enhance the development of biomarkers for the diagnosis and prognosis of ALS patients, increase our understanding of ALS pathogenesis, and shed light on the basis of ALS clinical heterogeneity. This work builds the foundation to realize personalized medicine strategies for ALS.
Author contributions
VPM wrote the manuscript and prepared the figures. CM is the CAPTURE ALS program manager and assisted with writing the manuscript. SK, ND, AG, JR, CVV and DT are members of the CAPTURE ALS executive committee and reviewed the manuscript in detail. SK, YY, CVV, ER, AE, RG, TB, JR WJ, AG, ND, KJ, LZ, YIM are members of the scientific committee. SK, AD, AG, ND and LZ are site principal investigators. HK reviewed the manuscript in detail. MB is a project manager at a CAPTURE ALS site. SD is the Project Manager and Software Developer at LORIS. JK is scientific director of C-BIG. All authors have read and approved the manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Fournier C, Glass JD. Modeling the course of amyotrophic lateral sclerosis. Nat Biotechnol. 2015;33:45–7.
- Kalra S, Khan MU, Barlow L, Beaulieu C, Benatar M, Briemberg H, et al. The Canadian ALS Neuroimaging Consortium (CALSNIC) – A multicentre platform for standardized imaging and clinical studies in ALS [Internet]. medRxiv. medRxiv; 2020. p. 2020.07.10.20142679.
- Ta D, Khan M, Ishaque A, Seres P, Eurich D, Yang YH, et al. Reliability of 3D texture analysis: a multicenter MRI study of the brain. J Magn Reson Imaging. 2020;51:1200–9.
- Kalra S, Müller HP, Ishaque A, Zinman L, Korngut L, Genge A, et al. A prospective harmonized multicenter DTI study of cerebral white matter degeneration in ALS. Neurology. 2020;95:e943–e952.
- Bharti K, Khan M, Beaulieu C, Graham SJ, Briemberg H, Frayne R, et al. Involvement of the dentate nucleus in the pathophysiology of amyotrophic lateral sclerosis: a multi-center and multi-modal neuroimaging study. NeuroImage Clin. 2020; 28: 102385
- Chenji S, Ishaque A, Mah D, Fujiwara E, Beaulieu C, Seres P, et al. Neuroanatomical associations of the Edinburgh cognitive and Behavioural ALS screen (ECAS). Brain Imaging Behav. 2021 Jun;15(3):1641–1654.
- Ta D, Ishaque A, Srivastava O, Hanstock C, Seres P, Eurich DT, et al. Progressive neurochemical abnormalities in cognitive and motor subgroups of amyotrophic lateral sclerosis: a prospective multicenter study. Neurology. 2021;97:e803–13–e813.
- Ishaque A, Ta D, Khan M, Zinman L, Korngut L, Genge A, et al. Distinct patterns of progressive gray and white matter degeneration in amyotrophic lateral sclerosis. Hum Brain Mapp 2021.
- Das S, Glatard T, Rogers C, Saigle J, Paiva S, Macintyre L, et al. Cyber infrastructure for open science at the Montreal neurological institute. Front Neuroinform. 2017; Jan 6;10:53
- Das S, Abou-Haidar R, Rabalais H, Sun SDLW, Rosli Z, Chatpar K, et al. The C-BIG repository: an institution-level open science platform. Neuroinform. 2021.
- Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:9–14.
- Niven E, Newton J, Foley J, Colville S, Swingler R, Chandran S, et al. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): a cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:172–9.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21.
- Srivastava O, Hanstock C, Chenji S, Mah D, Eurich D, Ta D, et al. Cerebral degeneration in amyotrophic lateral sclerosis: a prospective multicenter magnetic resonance spectroscopy study. Neurol Clin Pract. 2019;9:400–7.
- E Elahi GMM, Kalra S, Zinman L, Genge A, Korngut L, Yang YH. Texture classification of MR images of the brain in ALS using M-CoHOG: a multi-center study. Comput Med Imaging Graph 2020. Jan;79:101659
- Jadoul Y, Thompson B, de Boer B. Introducing Parselmouth: a Python interface to Praat. J Phon. 2018;71:1–15.
- Yunusova Y, Graham NL, Shellikeri S, Phuong K, Kulkarni M, Rochon E, et al. Profiling speech and pausing in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). PLOS One. 2016;11:e0147573.
- Yunusova Y, Green JR, Wang J, Pattee G, Zinman L. A protocol for comprehensive assessment of bulbar dysfunction in amyotrophic lateral sclerosis (ALS). J Vis Exp. 2011. Feb 21;(48):2422.
- Barnett C, Green JR, Marzouqah R, Stipancic KL, Berry JD, Korngut L, et al. Reliability and validity of speech & pause measures during passage reading in ALS. Amyotroph Lateral Scler Front Degener. 2020; Feb;21(1-2):42-50
- Wang J, Kothalkar PV, Kim M, Yunusova Y, Campbell TF, Heitzman D, et al. Predicting intelligible speaking rate in individuals with amyotrophic lateral sclerosis from a small number of speech acoustic and articulatory samples. 2016. Sep;2016:91-97
- Rong P, Yunusova Y, Wang J, Green JR. Predicting early bulbar decline in amyotrophic lateral sclerosis: a speech subsystem approach. Behav Neurol. 2015;2015:183027.
- Alas JK, Godlovitch G, Mohan CM, Jelinski SA, Khan AA. Regulatory Framework for Conducting Clinical Research in Canada. Can J Neurol Sci Le J Can des Sci Neurol. 2017 Sep;44(5):469–74.
- Johnson SB, Whitney G, McAuliffe M, Wang H, McCreedy E, Rozenblit L, et al. Using global unique identifiers to link autism collections. J Am Med Informatics Assoc. 2010. Nov-Dec 2010;17(6):689–95.
- Das S, Zijdenbos AP, Harlap J, Vins D, Evans AC. LORIS: a web-based data management system for multi-center studies. Front Neuroinform 2012Jan 20;5:37
- Das S, Lecours Boucher X, Rogers C, Makowski C, Chouinard-Decorte F, Oros Klein K, et al. Integration of “omics” data and phenotypic data within a unified extensible multimodal framework. Front Neuroinform 2018. Dec 18;12:91
- Mohaddes Z, Das S, Abou-Haidar R, Safi-Harab M, Blader D, Callegaro J, et al. National neuroinformatics framework for Canadian Consortium on Neurodegeneration in Aging (CCNA). Front Neuroinform 2018;12:85.
- Caspers S, Moebus S, Lux S, Pundt N, Schütz H, Mühleisen TW, et al. Studying variability in human brain aging in a population-based German cohort-rationale and design of 1000BRAINS. Front Aging Neurosci 2014;6:149.
- Tremblay-Mercier J, Madjar C, Das S, Pichet Binette A, Dyke SOM, Étienne P, PREVENT-AD Research Group, et al. Open science datasets from PREVENT-AD, a longitudinal cohort of pre-symptomatic Alzheimer's disease. Neuroimage Clin. 2021;31:102733.
- Das S, Zijdenbos AP, Harlap J, Vins D, Evans AC. LORIS: a web-based data management system for multi-center studies. Front Neuroinform. 2011;5:37.
- Van Rheenen W, Pulit SL, Dekker AM, Al Khleifat A, Brands WJ, Iacoangeli A, et al. Project MinE: study design and pilot analyses of a large-scale whole-genome sequencing study in amyotrophic lateral sclerosis. Eur J Hum Genet. 2018.
- Steinbach R, Gaur N, Stubendorff B, Witte OW, Grosskreutz J. Developing a neuroimaging biomarker for amyotrophic lateral sclerosis: multi-center data sharing and the road to a “global cohort.” Front Neurol 2018. Dec 4;9:1055
- Turner MR, Grosskreutz J, Kassubek J, Abrahams S, Agosta F, Benatar M, et al. Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:400–3.
- Müller HP, Turner MR, Grosskreutz J, Abrahams S, Bede P, Govind V, et al. A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:570–9.
- Farhan SMK, Bartha R, Black SE, Corbett D, Finger E, Freedman M, et al. The Ontario Neurodegenerative Disease Research Initiative (ONDRI). Can J Neurol Sci 2017. Mar;44(2):196-202.
- Ramirez J, Holmes MF, Scott CJM, Ozzoude M, Adamo S, Szilagyi GM, et al. Ontario Neurodegenerative Disease Research Initiative (ONDRI): structural MRI methods and outcome measures. Front Neurol 2020. Aug 11;11:847
- McLaughlin PM, Sunderland KM, Beaton D, Binns MA, Kwan D, Levine B, et al. The quality assurance and quality control protocol for neuropsychological data collection and curation in the Ontario Neurodegenerative Disease Research Initiative (ONDRI) Study. Assessment 2021;28:1267–86.
- Kapoor A, Bartha R, Black SE, Borrie M, Freedman M, Gao F, et al. Structural brain magnetic resonance imaging to rule out comorbid pathology in the assessment of Alzheimer’s disease dementia: findings from the Ontario Neurodegenerative Disease Research Initiative (ONDRI) Study and clinical trials over the past 10 years. J Alzheimer’s Dis 2020;74(3):747–757.
