ABSTRACT
Introduction: Non-cystic fibrosis bronchiectasis (NCFB) is an increasingly prevalent chronic respiratory disease, characterized by a cycle of infection and inflammation. It is progressive and associated with poor quality of life, increasing healthcare costs and mortality. Inhaled antibiotics offer the potential to decrease long-term bacterial burden and reduce or delay exacerbations which are a key driver of disease progression and healthcare costs. Currently however, no inhaled treatments are approved for the prevention of exacerbations in patients with NCFB.
Areas covered: We consider current evidence for inhaled treatment in NCFB and discuss ciprofloxacin dry powder for inhalation (DPI), a novel treatment using PulmoSphere™ technology, delivered via a pocket-sized, breath-actuated inhaler. We also detail the unique features of the ongoing Phase 3 RESPIRE trials for ciprofloxacin DPI.
Expert opinion: The simplicity of use and short administration time could make ciprofloxacin DPI an attractive treatment option with potential to reduce the number of exacerbations in patients with NCFB. The RESPIRE studies are the largest carried out in NCFB to date and will provide a benchmark for future trial design. If approved, following successful Phase 3 data, ciprofloxacin DPI has potential for significant use in NCFB due to its good tolerability and low treatment burden.
1. Introduction
Non-cystic fibrosis bronchiectasis (NCFB) is a chronic respiratory disease characterized by permanent and abnormal dilation of the bronchi leading to cough, purulent sputum and episodic infective exacerbations [Citation1–Citation3]. It is a progressive disease that results in thickening of the bronchial wall which worsens with time [Citation2,Citation3]. NCFB is associated with a poor quality of life and increased healthcare utilization and cost [Citation4–Citation6]. NCFB represents a heterogeneous respiratory pathology with multiple etiologies. Postinfectious disease is frequently reported [Citation7], however, up to 53% of cases still have an idiopathic origin [Citation2,Citation7,Citation8].
The pathophysiology of NCFB is modeled on the cycle hypothesis, [Citation9]; the exacerbations and chronic respiratory infection that underlie the cycle are key drivers associated with disease progression, mortality, and poor quality of life [Citation10–Citation14]. Disrupting different aspects of the cycle has been the primary treatment approach to date [Citation2,Citation8,Citation15]. Given that around 40% of NCFB patients suffer from three or more exacerbations per year, a large proportion of patients may benefit from strategies to reduce the frequency of exacerbations [Citation16].
Figure 1. The vicious cycle of inflammation and infection and potential therapeutic interventions [Citation9]. Reproduced with permission of the European Respiratory Society ©. Eur J Respir Dis Suppl. 1986; 147: 6–15.
![Figure 1. The vicious cycle of inflammation and infection and potential therapeutic interventions [Citation9]. Reproduced with permission of the European Respiratory Society ©. Eur J Respir Dis Suppl. 1986; 147: 6–15.](/cms/asset/9f81d82c-adf3-40bf-a975-ed31bcd4f50f/ieod_a_1196129_f0001_b.gif)
Historically, NCFB prevalence has been poorly described [Citation15] and the exact incidence remains unclear. However, some consistent themes can be observed in data from the US, Europe and Asia Pacific (); overall the prevalence of bronchiectasis is increasing and rates are particularly high in the elderly. Reports of prevalence have ranged from 4.2 per 100,000 in 18–34 year old people in the US [Citation5], to as high as 135 per 10,811 in China [Citation17] and recently, Quint et al. reported an overall point prevalence of 125.74 per 100,000 in UK primary care patients over the age of 70 [Citation18]. Data from large registries are awaited to gain a greater knowledge of the prevalence of bronchiectasis. Data suggests wide variation in prevalence (, [Citation5,Citation17–Citation20]).
Table 1. The exact prevalence of bronchiectasis is unclear and there are differing reports across the US, Europe and Asia Pacific.
2. Overview of the market
2.1. What are the current strategies being applied to tackle the unmet need?
Current treatment guidelines recommend a number of approaches to managing NCFB, including airway clearance, airway drug therapy (primarily bronchodilators) and long-term antibiotic use. Surgery may be indicated in highly selected cases with localized disease [Citation2]. The rationale for using antibiotics to reduce or delay exacerbations is based on long-term reduction of bacterial burden and the subsequent reduction of inflammation. By lowering bacterial burden, there is a dampening of chronic inflammation and resultant tissue damage. Consequently, if the number of exacerbations is reduced, a concurrent improvement should occur in a patient’s symptoms [Citation8,Citation21]. Inhaled therapies that directly target the site of infection enable high drug doses to be delivered, while minimizing systemic exposure [Citation22]. Unfortunately, there is a paucity of high-quality evidence for the use of inhaled therapies in NCFB and guidelines recommending the use of inhaled antibiotics are based on data graded as poor quality from small studies, or from larger Phase III clinical studies that did not meet their primary end point. Furthermore, as these studies have all used different end points, outcomes cannot be directly compared.
2.2. Which competitor compounds/classes of compounds are in the clinic/late development?
A small (n = 65) single-blinded, controlled study of nebulized gentamicin versus nebulized 0.9% saline, showed antibiotic therapy significantly reduces the density of sputum bacteria in patients with NCFB, as well as improving exercise capacity and reducing exacerbations. The authors concluded however, that therapy needed to be continuous to maintain efficacy [Citation23]. Other recent trials of therapies aiming to reduce exacerbations, such as the non-antibiotic mucoactive inhaled mannitol [Citation24,Citation25], or nebulized antibiotics such as colistin [Citation26], and aztreonam lysine [Citation27] all failed to show significant benefits in their primary end points. This may reflect the choice of end point(s) or the prerequisite study population rather than a true lack of efficacy. Finally, a small Phase II study (n = 42) of dual-release ciprofloxacin delivered via nebulization indicated antipseudomonal activity and a delay in time to first exacerbation [Citation28]; Phase III trials are ongoing [Citation29,Citation30]. summarizes ongoing and recent clinical trials of inhaled antibiotics in NCFB.
Table 2. Ongoing and recent clinical trials of inhaled antibiotics in NCFB.
It is clear that there is a significant unmet need for well-designed trials in appropriate patient populations to establish the efficacy and safety of inhaled antibiotic therapies, particularly in patients with moderate or severe disease.
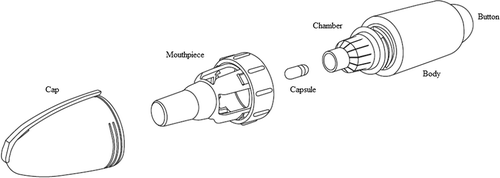
3. Introduction to ciprofloxacin dry powder for inhalation (DPI) ()
Ciprofloxacin is a broad-spectrum fluoroquinolone antibiotic with proven bactericidal efficacy against a range of pathogens seen in NCFB, including P. aeruginosa [Citation36]. It is currently indicated for the treatment of systemic infections including respiratory infections [Citation36]. Since becoming available, approximately 522 million patients have been treated with oral or intravenous formulations of Cipro® (Bayer; data on file, Bayer), the drug therefore has a well-established safety profile [Citation37]. The activity of ciprofloxacin is concentration dependent: the higher the peak concentration, the higher the bactericidal activity. This makes it a particularly suitable candidate for an inhaled product, where high drug concentrations can be achieved in the lung.
Box 1. Drug summary
Ciprofloxacin DPI is in development as a long-term intermittent therapy to reduce the frequency of acute exacerbations in NCFB patients with respiratory bacterial pathogens [Citation38]. Patients inhale ciprofloxacin 32.5 mg inhalation powder from one capsule twice-daily using a pocket sized inhaler. To administer the drug patients insert a single capsule then inhale deeply, this breath is then held for an additional five seconds. The small delivery device is easily understood and is designed to be both user-friendly and portable as it does not require a power source or cleaning. Furthermore it has a short administration time, with the drug usually delivered in one breath. Together these qualities mean the drug/device combination avoids many of the problems associated with nebulizers such as time-consuming administration [Citation39], complex cleaning, sterilization and maintenance, and a decrease in the efficiency of drug delivery over time [Citation40]. The device is already approved for the delivery of tobramycin and used in some countries for the treatment of cystic fibrosis patients in the form of the TOBI® Podhaler™ (Novartis) [Citation41,Citation42]
Ciprofloxacin DPI () is undergoing Phase III trials in patients with idiopathic or post-infectious NCFB, to assess its efficacy in reducing exacerbations in patients with evidence of respiratory pathogens who suffer from two or more exacerbations annually [Citation31,Citation32].
4. Chemistry
Ciprofloxacin DPI consists of aerosolized ciprofloxacin that has been spray dried using the PulmoSphere™ spray drying process [Citation38]. The technology creates a dry phospholipid ‘shell’ around the ciprofloxacin core which is thought to disperse rapidly upon introduction to the lung thus exposing the ciprofloxacin crystals. This may improve the tolerability of the drug, potentially reducing bronchospasm or cough. The process also facilitates controllable and reproducible particle size, density, morphology, and surface composition, all of which are critical to ensuring efficient and reproducible dose delivery to the lungs [Citation38]. The active ingredient in ciprofloxacin DPI is ciprofloxacin hydrated (sometimes referred to as betaine). This form has lower solubility than the HCl salt which is rapidly absorbed through lung tissue; the ciprofloxacin hydrated form allows for a longer residence time in the lungs [Citation43].
A total of 50 mg of ciprofloxacin DPI (equivalent to 32.5 mg of ciprofloxacin) is delivered in each dose; the small particles (≤5 µm mass median aerodynamic diameter) are optimized for delivery into the bronchopulmonary system and the mode of administration ensures high concentrations of the drug reach the lungs [Citation44].
5. Pharmacodynamics, pharmacokinetics and metabolism
Phase I studies assessing the pharmacodynamics (PD) and pharmacokinetics (PK) of ciprofloxacin DPI have been carried out in healthy volunteers, and patients with cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD). These studies showed that ciprofloxacin DPI attains high pulmonary concentrations coupled with low systemic exposure [Citation45–Citation49]. Concentrations of the drug in induced sputum are considerably higher than plasma levels; in a study of patients with CF, ciprofloxacin DPI (single dose, 32.5 mg) achieved a Cmax (mg/L) of 34.9 and 0.0790 and an AUC (mg•h/L) of 89.5 and 0.425 in sputum and plasma respectively [Citation50]. Preclinical studies in vivo also show that ciprofloxacin DPI has a higher t1/2 vs. the soluble ciprofloxacin hydrochloride (HCl) suggesting that it has prolonged residence in the lung [Citation43]. Ciprofloxacin HCl has a serum half-life of approximately 4 h [Citation36] while ciprofloxacin DPI terminal half-life in healthy volunteers was found to be 9.5 h in a Phase I study [Citation44]. Further key pharmacokinetic properties are summarized in .
Table 3. Key pharmacokinetic/pharmacodynamics properties of ciprofloxacin DPI in plasma and sputum.
Lung deposition data are available for healthy volunteers and patients with NCFB, COPD and CF. Data from scintigraphic studies confirm that ciprofloxacin DPI is delivered in high and reproducible doses to the entire lung with minimal amounts of drug remaining in the device following administration [Citation51]. Physiological modeling in healthy volunteers indicates that approximately 40% of the inhaled dose reaches the lower respiratory tract [Citation44,Citation51]. This is high in comparison to the drug deposition achieved by other DPI products currently on the market; for example Colobreathe® Turbospin® (Forest Laboratories) which delivered on average 11.9% and 11.6% to the whole lung with or without pretreatment with salbutamol respectively [Citation52]. Ciprofloxacin DPI therefore has the potential to be one of the highest delivering systems to date, with good distribution to the airways and the distal airways/alveoli, which we will collectively refer to as ‘delivery to the lung’ for simplicity.
6. Clinical efficacy
6.1. Phase II studies
Phase II studies in patients with CF indicated that ciprofloxacin DPI reduced bacterial load but did not significantly improve FEV1 [Citation53]. This may have been due to several reasons including the advanced nature of disease in the study population [Citation22,Citation54].
In a Phase II randomized, placebo-controlled, double-blind study of ciprofloxacin DPI in NCFB, adult patients with post-infectious or idiopathic NCFB with positive culture for P. aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Enterobacteriaceae, Stenotrophomonas maltophilia or Achromobacter xylosoxidans were enrolled and received either ciprofloxacin DPI 32.5 mg (n = 60) or matching placebo (n = 64) dispensed from a T-326 inhaler for 28 days and were followed for a further 56 days after end of therapy [Citation55].
The results of the Phase II study are the first in NCFB to demonstrate that ciprofloxacin DPI produces a statistically significant reduction in bacterial load. Ciprofloxacin DPI had a potent bactericidal action and led to a significantly greater mean reduction in colony forming unit (CFU) count vs placebo at the end of therapy, equal to a 3.62 log reduction in bacterial load. Bacterial eradication also occurred at significantly higher rates in the ciprofloxacin DPI arm vs. placebo at day 8 (20/42, 48% vs. 6/51, 12%; p < 0.001) and end of therapy (14/40, 35% vs. 4/49, 8% p = 0.001) () [Citation55].
Figure 3. Phase II study results: significant reductions in bacterial load were seen for patients treated with ciprofloxacin DPI [Citation55]. Mean bacterial load for the modified intent-to-treat population treated with ciprofloxacin DPI in the Phase II study. Shaded area indicates treatment period (days). Mean reductions in colony forming units at end of therapy were significantly higher ciprofloxacin DPI vs placebo: −3.62 log10 CFU·g−1, range −9.78–5.02 log10 CFU·g−1 vs −0.27 log10 CFU·g−1, range −7.96–5.25 log10 CFU·g−1 *** p = 0.001. Reproduced with permission of the European Respiratory Society ©: European Respiratory Journal May 2013, 41 (5) 1107‐1115; Doi: 10.1183/09031936.00071312.
![Figure 3. Phase II study results: significant reductions in bacterial load were seen for patients treated with ciprofloxacin DPI [Citation55]. Mean bacterial load for the modified intent-to-treat population treated with ciprofloxacin DPI in the Phase II study. Shaded area indicates treatment period (days). Mean reductions in colony forming units at end of therapy were significantly higher ciprofloxacin DPI vs placebo: −3.62 log10 CFU·g−1, range −9.78–5.02 log10 CFU·g−1 vs −0.27 log10 CFU·g−1, range −7.96–5.25 log10 CFU·g−1 *** p = 0.001. Reproduced with permission of the European Respiratory Society ©: European Respiratory Journal May 2013, 41 (5) 1107‐1115; Doi: 10.1183/09031936.00071312.](/cms/asset/e3ae05bb-81d8-41a7-9398-4c68b73b2869/ieod_a_1196129_f0003_b.gif)
Some increases in minimum inhibitory concentrations were seen in the ciprofloxacin DPI arm for P. aeruginosa (n = 4), S. maltophilia (n = 1) and H. influenzae (n = 1) but most were transient and decreased to susceptible levels by the end of the study. A trend toward improved quality of life was also seen at the end of treatment, with an adjusted mean difference in St Georges Respiratory Questionnaire (SGRQ) score for ciprofloxacin DPI compared with placebo of −3.56 (95% CI −7.3 to 0.1; p = 0.059). A concurrent improvement of four-points in the SGRQ (i.e. a clinically significant improvement) was also seen in 40% of patients receiving ciprofloxacin DPI vs. 32% in patients receiving placebo. Overall this study provided evidence that targeted delivery of ciprofloxacin DPI resulted in a clear reduction in bacterial load and was well tolerated, with potential to improve quality of life (QoL) outcomes. Following these positive results ciprofloxacin DPI moved into Phase III development.
6.2. Phase III studies
6.2.1. Study design
The fully enrolled, ongoing Phase III trials of ciprofloxacin DPI in NCFB – RESPIRE 1 and RESPIRE 2 – represent the largest program to be carried out in NCFB to date, with a planned study population of over 900 patients [Citation31,Citation32,Citation56,Citation57]. The two studies are of the same design: international prospective, parallel-group, randomized, double-blinded, multicenter, placebo-controlled trials scheduled to run for 48 weeks with an 8-week follow-up period after the last dose [Citation56,Citation57].
In both studies, patients follow a pattern of 28 days on- and 28 days off-drug, resulting in a total of six active cycles; or a pattern of 14 days on- and 14 days off-drug resulting in a total of 12 active cycles (). These two patterns were chosen because, although a 28-day cycle is widely used in CF treatment, there is no evidence for the appropriate cycle duration in patients with NCFB; therefore the two treatment regimens are being studied with the aim of providing novel scientific insights into optimal treatment durations for NCFB. In both studies, patients with NCFB are scheduled to receive cyclical regimens of twice-daily ciprofloxacin DPI 32.5 mg or matching placebo.
Figure 4. RESPIRE 1 and 2 treatment regimens [Citation56].
![Figure 4. RESPIRE 1 and 2 treatment regimens [Citation56].](/cms/asset/0d328b2b-f614-4b29-a20c-70f0971f99de/ieod_a_1196129_f0004_b.gif)
6.2.2. Inclusion and exclusion criteria
Taken in their entirety, the inclusion/exclusion criteria help ensure that the patients enrolled are representative of the population most likely to benefit from long-term, cyclical use of ciprofloxacin DPI.
The strict entry criteria for RESPIRE 1 and 2 aim to ensure that the study population only includes patients with either postinfectious or idiopathic disease, rather than a heterogeneous population as seen in previous studies () [Citation58]. Inclusion is also restricted to patients who have two or more exacerbations in the prior 12 months and are therefore most in need of a therapy that reduces exacerbation frequency. The program is designed to take into account the microbiological diversity found in patients with NCFB; respiratory pathogens infect the bronchial airways in a large number of those with the disease and recent data highlight the consequences of chronic bacterial infection on patient outcomes including increasing hospital admission and mortality rates [Citation10,Citation15,Citation59]. Although P. aeruginosa is a known prognostic marker for poor outcomes, including a three-fold increase in mortality as well as increased risk of exacerbations [Citation60], there is evidence that other common pathogens such as H. influenzae and S. aureus also have negative consequences [Citation10,Citation59,Citation61]. P. aeruginosa isolation rates vary depending on the setting and may be subject to tertiary care center bias. In the US bronchiectasis registry, 37% Pseudomonas species isolation was reported [Citation62] and recently EMBARC (European Multicentre Bronchiectasis Audit and Research Collaboration) reported that P. aeruginosa was isolated in 290 of 1283 patients [Citation63]. A large proportion of patients are also colonized with pathogens other than P. aeruginosa and therefore RESPIRE was designed to enroll patients who have at least one of seven predefined pathogens – P. aeruginosa, H. influenzae, M. catarrhalis, S. aureus, S. pneumoniae, S. maltophilia and Burkholderia cepacia.
Table 4. Key inclusion and exclusion criteria for the RESPIRE clinical trial program.
6.2.3. End points
Two end points, developed in conjunction with the regulatory authorities, are being used in the RESPIRE studies (): time to first exacerbation and frequency of exacerbation during the 48-week study. Exacerbations are strictly defined as requiring systemic antibiotic use, and worsening of at least three signs or symptoms and fever or malaise/fatigue. This stringent definition aims to prevent symptoms that are caused by, for example respiratory viruses, from being mislabeled as bacterial exacerbations. Other end points include: frequency of exacerbations requiring systemic antibiotics and at least one sign or symptom; quality of life and microbiology end points, lung function and safety.
Table 5. Main end points in the RESPIRE clinical trial program.
6.2.4. Analyses: patient stratification
Patients are stratified for 2 : 1 (ciprofloxacin DPI : placebo) randomization according to geographic region, positive culture for P. aeruginosa and chronic (≥6 months) macrolide use. As a number of NCFB patients in day-to-day clinical practice receive chronic macrolide therapy [Citation16,Citation64], the efficacy and safety of any new therapies that may be used in conjunction with this should be assessed, as in the RESPIRE cohorts. Furthermore given that patients chronically infected with P. aeruginosa have relatively high rates of persistent infection and hospitalization [Citation59], and that ciprofloxacin has potent antipseudomonal properties it will be important to ascertain whether any benefits from the drug are dependent on the presence or absence of this pathogen.
7. Safety and tolerability
The safety profile of ciprofloxacin DPI has been assessed in several studies, and the drug has been shown to be generally well tolerated. Several Phase I and II trials indicate that ciprofloxacin DPI is generally well tolerated in patients with COPD, CF or NCFB and results from Phase III studies are awaited to confirm this [Citation49–Citation51,Citation53,Citation55]. Treatment emergent adverse events (AEs) in Phase II studies have been similar between ciprofloxacin DPI- and placebo-treated patients both in nature and frequency. Most commonly, ciprofloxacin DPI patients have reported abnormal product taste/dysgeusia which is characteristic of the drug [Citation53,Citation55]. The low occurrence of respiratory tract irritation in studies of ciprofloxacin DPI is particularly noteworthy as cough, bronchospasm and bronchial obstruction pose potential tolerability issues for current and future inhaled therapies. In a recent trial of aztreonam for inhalation solution, cough and bronchospasm were reported as serious AEs in 1/134 and 3/134 of aztreonam patients respectively [Citation33] and cough reported amongst other AEs in 66/135 patients [Citation34]. Of a total of 153 patients treated with ciprofloxacin DPI in two studies (one in NCFB and the other CF), bronchospasm was reported in only six patients overall [Citation53,Citation55]. Cough occurrence in ciprofloxacin DPI- and placebo-treated patients in the CF study was 3.2% and 10.8% respectively [Citation53] and in the NCFB study this rate was 0% and 7.8% [Citation55]. This compares favorably to other inhaled dry powder products such as the TOBI Podhaler where cough is perceived to be problematic [Citation65].
8. Regulatory affairs
In April 2014, the US Food and Drug Administration’s (FDA) Office of Orphan Products Development granted Orphan Drug Designation (ODD) to ciprofloxacin DPI for the treatment of NCFB. In addition to ODD, in November 2014 the FDA also granted ciprofloxacin DPI Qualified Infectious Disease Product (QIDP) status. Antimicrobial drugs which are designed to treat serious and life-threatening infections and are designated as QIDP are eligible for fast-track designation, priority review by the FDA, and a five-year extension of market exclusivity.
9. Conclusions
NCFB is an increasingly prevalent respiratory pathology associated with significant morbidity and mortality. Inhaled antibiotic therapies that directly target the site of infection may provide effective treatment options to reduce and delay exacerbations and improve quality of life, however there are currently no such licensed products. Ciprofloxacin DPI is an inhaled formulation of an antibiotic with proven efficacy against a range of respiratory pathogens, and is currently in Phase III development. Lung tolerability data suggest that ciprofloxacin DPI has the potential to reduce adverse events, such as cough and bronchospasm, which are perceived to be common with other inhaled antibiotics in this patient population. The current clinical trial program is the largest interventional trial in NCFB with one of the most robust study designs including patients with a variety of common respiratory pathogens, including P. aeruginosa. The inclusion of patients infected with P. aeruginosa and non-P. aeruginosa bacteria aims to increase our understanding of the likely clinical impact of these pathogens. It is hoped that these studies will provide new treatment options for patients suffering from this debilitating disease.
10. Expert opinion
10.1. What, if any, improvement does the drug/therapy hold over other therapies?
Ciprofloxacin DPI is delivered directly to the lungs in as little as one breath without the need for nebulization. Unlike many nebulized formulations, the PulmoSphere technology that carries ciprofloxacin DPI is aerodynamic and optimized for delivery into the bronchopulmonary system thus ensuring high concentrations of the drug reach the distal lung. The dry powder form offers good tolerability with no significant cough or bronchospasm adverse events reported in Phase I and II trials. The simplicity and short administration time make ciprofloxacin DPI an attractive treatment option in comparison to nebulized delivery. Furthermore, by targeting the lung, high drug concentrations in the airways are obtained and these are coupled with low systemic exposure; this could decrease the risk of systemic adverse events and potentially reduce the development of resistance in some cases, though long-term data are needed to establish this.
10.2. In developing this drug, what are the key lessons for R&D scientists in the field? Could these lessons be applied to other rare diseases/niche indications?
The RESPIRE studies will provide a benchmark for clinical trial design in NCFB. The etiological entry criteria used greatly broadens the applicability of the study results. The assessment of a monthly and 2-weekly treatment schedule is distinct and unique to the RESPIRE studies and is the first time such a design has been used in trials for NCFB. Furthermore stratifying patients by P. aeruginosa presence or macrolide use may provide valuable insights into ‘responder populations’. As the studies have no restrictions on the baseline MICs of pathogens at the time of enrolment, the ability of the drug to treat patients with resistant pathogens will be elucidated.
10.3. What, if any, impact is this drug/therapy likely to have on current treatment strategies?
There is currently no approved inhaled antibiotic therapy for NCFB and the off-label use of inhaled antibiotics with no evidence base is common but likely unsustainable [Citation16]. Robust efficacy data emerging from the RESPIRE trials is likely to improve clinical access. The simplicity of ciprofloxacin DPI use may increase the overall proportion of NCFB patients on inhalational antibiotics.
10.4. How likely are physicians to prescribe the drug?
Upon successful Phase III trials it is anticipated that there will be significant usage of this drug. The tolerability and low treatment burden coupled with the robust evidence expected make it an attractive option with advantages over current off-label NCFB treatments, to physicians, patients and payers alike. Ciprofloxacin DPI has potential to be used in both specialist and general respiratory secondary care clinics.
10.5. What data are still needed?
To be widely embraced, cost-effectiveness studies and better understanding of emergence and implications of ciprofloxacin resistance during prolonged treatment will be needed. Current studies are not powered to show mortality benefits and such data, perhaps in postmarketing observational studies, will be of great interest to clinicians. Furthermore, studies to determine ‘eradication’ of Pseudomonas in the long-term will be important.
Physicians will also wish to see ciprofloxacin DPI studies in more diverse phenotypes of bronchiectasis: current RESPIRE studies include around 40–60% of potential etiological causes of bronchiectasis [Citation2,Citation59]. Many patients also present with moderate to severe COPD and this bronchiectasis–COPD overlap [Citation66] is associated with a poorer prognosis compared to idiopathic bronchiectasis [Citation12]. It is estimated that bronchiectasis–COPD overlap syndrome (BCOS) may affect as many as 7 million patients in the EU alone [J Chalmers personal communication] and therefore studies of ciprofloxacin DPI in BCOS will be of great interest.
10.6. Where is this drug likely to be in 5 years’ time?
As ciprofloxacin DPI is still undergoing Phase III trials, it has not yet been approved and full efficacy and safety data are still required. If the drug reaches its primary end point in the RESPIRE studies, it is likely to be in widespread use in the future – possibly continuously or in milder cases covering the winter exacerbation period. It should be noted however that, as the drug is not currently approved, these uses may fall outside any future license.
Declaration of interest
Writing support was provided by highfield:communication, Oxford, UK and funded by Bayer Pharma AG. The manuscript was derived and completed independently of Bayer, but the final manuscript was assessed by Bayer for accuracy. A De Soyza has received speakers fees, travel/congress attendance support from AstraZeneca, Bayer, Chiesi, GlaxoSmithKline, Forrest and Novartis, and has received research grants from Bayer, Forrest and Novartis. T Aksamit has participated in trials sponsored by Bayer, Aradigm/Grifols, Insmed, and Gilead but has not received personal or research support. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- O’Donnell AE. Bronchiectasis. Chest. 2008;134:815–823.
- Pasteur MC, Bilton D, Hill AT; British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):1–58.
- King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis. 2009;4:411–419.
- Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993–2006. Chest. 2010;138(4):944–949.
- Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12(4):205–209.
- Joish VN, Spilsbury-Cantalupo M, Operschall E. Economic burden of non-cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Appl Health Econ Health Policy. 2013;11(3):299–304.
- Lonni S, Chalmers JD, Goeminne PC, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc. 2015;12(12):1764–1770.
- McShane PJ, Naureckas ET, Tino G, et al. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188:647–656.
- Cole PJ. Inflammation: a two-edged sword – the model of bronchiectasis. Eur J Respir Dis. 1986;Suppl 147:6–15.
- Chalmers JD, Goeminne P, Aliberti S, et al. The Bronchiectasis Severity Index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585.
- Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J. 2009;34:843–849.
- Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med. 2014;108:287–296.
- Martínez-García MA, Soler-Cataluña J-J, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007;132(5):1565–1572.
- McDonnell M, Goeminne P, Alberti S, et al. Comparative analysis of the predictive utility of clinical disease severity scores for non-cystic fibrosis bronchiectasis. Eur Resp J. 2014;44(Suppl 58) [cited 2016 Mar 14]. Available from: http://erj.ersjournals.com/content/44/Suppl_58/P2484
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J. 2015;45:1446–1462.
- Hill AT, Routh C, Welham S. National BTS bronchiectasis audit 2012: is the quality standard being adhered to in adult secondary care? Thorax. 2014;69:292–294.
- Zhou Y-M, Wang C, Yao W-Z, et al. [The prevalence and risk factors of bronchiectasis in residents aged 40 years old and above in seven cities in China]. Zhonghua Nei Ke Za Zhi. 2013;52(5):379–382. Chinese.
- Quint JK, Millett ERC, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004–2013: a population based cohort study. Eur Respir J. 2015;47(1):186–193.
- Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439.
- Ringshausen FC, De Roux A, Diel R, et al. Bronchiectasis in Germany: a population based estimation of disease prevalence. Eur Respir J. 2015;46(6):1805–1807.
- O’Donnell AE. Bronchiectasis: which antibiotics to use and when? Curr Opin Pulm Med. 2015;21:272–277.
- Weers J. Inhaled antimicrobial therapy – barriers to effective treatment. Adv Drug Deliv Rev. 2014;85:24–43.
- Murray MP, Govan JR, Doherty CJ, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2011;183(4):491–499.
- Bilton D, Daviskas E, Anderson SD, et al. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non-cystic fibrosis bronchiectasis. Chest. 2013;144:215–225.
- Bilton D, Tino G, Barker AF, et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial. Thorax. 2014;69:1073–1079.
- Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2014;189:975–982.
- Barker AF, O’Donnell AE, Flume P, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised, double-blind, placebo-controlled phase 3 trials. Lancet Respir Med. 2014;2:738–749.
- Serisier DJ, Bilton D, De Soyza A, et al. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax. 2013;68:812–817.
- National Institutes of Health. 2016. Phase 3 study with dual release ciprofloxacin for inhalation in NCFB (ORBIT-3) [cited 2016 Mar 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT01515007
- National Institutes of Health. 2016. Phase 3 study with dual release ciprofloxacin for inhalation in NCFB (ORBIT-4) [cited 2016 Mar 9]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT02104245
- National Institutes of Health. 2016. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis (non-CF BE) (RESPIRE 1) [cited 2016 Mar 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT01764841
- National Institutes of Health. 2016. Ciprofloxacin dry powder for inhalation (DPI) in non-cystic fibrosis bronchiectasis (non-CF BE) (RESPIRE 2) [cited 2016 Mar 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT02106832
- AIR-BX1. Safety and effectiveness of AZLI (an inhaled antibiotic) in adults with non-cystic fibrosis bronchiectasis [cited 2016 Mar 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT01313624?term=NCT01313624&rank=1
- AIR-BX2. Safety and effectiveness of AZLI (an inhaled antibiotic) in adults with noncystic fibrosis bronchiectasis [cited 2016 Mar 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT01314716?term=NCT01314716&rank=1
- Inhaled Promixin® in the treatment of noncystic fibrosis bronchiectasis [cited 2016 Mar 10]. Available from: http://www.controlled-trials.com/ISRCTN49790596
- Ciprofloxacin summary of product characteristics [cited 2016 Mar 9]. Available from: https://www.medicines.org.uk/emc/medicine/20346/SPC/Ciproxin+Tablets+500mg/#PHARMACODYNAMIC_PROPS
- Ciprofloxacin European Medicines Agency report, annex 1. List of the names, pharmaceutical forms, strengths of the medicinal products, routes of administration, marketing authorisation holders in the member states [cited 2016 Mar 6]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Ciprofloxacin_Bayer_30/WC500008075.pdf
- Weers JG, Tarara TE. The PulmoSphere™ platform for pulmonary drug delivery. Ther Deliv. 2014;5:277–295.
- Quon BS, Goss CH, Ramsey BW. Inhaled antibiotics for lower airway infections. Ann Am Thorac Soc. 2014;11(3):425–434.
- Alhaddad B, Smith FJ, Robertson T, et al. Patients’ practices and experiences of using nebuliser therapy in the management of COPD at home. BMJ Open Resp Res. 2015. doi:10.1136/bmjresp-2014-000076
- Geller DE, Weers J, Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere technology. J Aerosol Med Pulm Drug Deliv. 2011;24:175–182.
- Lam J, Vaughan S, Parkins MD. Tobramycin inhalation powder (TIP): an efficient treatment strategy for the management of chronic Pseudomonas aeruginosa infection in cystic fibrosis. Clin Med Insights Circ Resp Pulm Med. 2013;7:61–77.
- Endermann R, Labischinski H, Ladel C, et al. Treatment of bacterial diseases of the respiratory organs. US20040254194 A1 (2004)
- Stass H, Nagelschmitz J, Willmann S, et al. Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: a Phase I study. Clin Drug Investig. 2013;33:419–427.
- Stass H, Badorrek P, Hohlfeld J, et al. Safety and pharmacokinetics of multiple-dose ciprofloxacin dry powder for inhalation in patients with moderate or severe chronic obstructive pulmonary disease. Presented at the European Respiratory Society Annual Congress; 2011 Sep 24–28; Amsterdam, The Netherlands; [Poster 3011].
- Tokimatsu I, Hiramatsu K, Morimoto T, et al. Safety, tolerability, and pharmacokinetics of a single dose of ciprofloxacin dry powder for inhalation (ciprofloxacin DPI) in Japanese patients with mild to moderate chronic obstructive pulmonary disease: a randomized controlled trial. Presented at the International Conference of the American Thoracic Society; 2011 May 13–18; Denver, CO, USA; [Abstract 3106].
- Kadota J, Tokimatsu I, Hiramatsu K, et al. A randomized controlled study to investigate the safety and pharmacokinetics of multiple doses of ciprofloxacin dry powder for inhalation in Japanese patients with moderate to severe COPD. Presented at the International Conference of the American Thoracic Society; 2012 May 18–23; California, CA, USA; [Poster 30923].
- Stass H, Nagelschmitz J, Watz H, et al. Safety and pharmacokinetics of two dose strengths of ciprofloxacin dry powder for inhalation (DPI) in patients with moderate to severe COPD. Presented at the European Respiratory Society Annual Congress; 2012 Sep 3; Vienna, Austria; [Abstract 2817].
- Stass H, Weimann B, Nagelschmitz J, et al. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a phase I, randomized, dose-escalation study. Clin Ther. 2013;35:1571–1581.
- Stass H, Delesen H, Nagelschmitz J, et al. Safety and pharmacokinetics of ciprofloxacin dry powder for inhalation in cystic fibrosis: a phase I, randomized, single-dose, dose-escalation study. J Aerosol Med Pulm Drug Deliv. 2015;28:106–115.
- Stass H, Nagelschmitz J, Kappeler D, et al. Lung deposition of ciprofloxacin dry powder for inhalation in healthy subjects and patients suffering from chronic obstructive pulmonary disease or non-cystic fibrosis bronchiectasis. Presented at the International Conference of the American Thoracic Society; 2013 May 19; Philadelphia, PA, USA; [Abstract 1507].
- Schwarz C. Colobreathe for the treatment of cystic fibrosis-associated pulmonary infections. Pulm Ther. 2015;1:19–30.
- Dorkin HL, Staab D, Operschall E, et al. Ciprofloxacin DPI: a randomised, placebo-controlled, phase IIb efficacy and safety study on cystic fibrosis. BMJ Open Respir Res. 2015. eCollection 2015. doi:10.1136/bmjresp-2015-000100
- Elborn JS. Ciprofloxacin dry powder inhaler in cystic fibrosis. BMJ Open Resp Res. 2016;3. doi:10.1136/bmjresp-2015-000125
- Wilson R, Welte T, Polverino E, et al. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a phase II randomised study. Eur Respir J. 2013;41:1107–1115.
- Aksamit T, De Soyza A, Operschall E, et al. A comparison of study designs of inhaled agents in non-cystic fibrosis bronchiectasis (NCFB): key differences in the phase 3 RESPIRE trials of ciprofloxacin dry powder for inhalation (DPI). Chest. 2015. doi:10.1378/chest.2277297
- De Soyza A, Aksamit T, Operschall E, et al. LATE-BREAKING ABSTRACT: baseline demographic profile of subjects of the phase 3 RESPIRE 1 trial of ciprofloxacin dry powder for inhalation (DPI) in non-cystic fibrosis bronchiectasis (NCFB). Eur Resp J. 2015;46(Suppl 59). doi:10.1183/13993003.congress-2015.PA2617
- Chalmers JD, Aliberti S, Polverino E, et al. The EMBARC European bronchiectasis registry: protocol for an international observational study. ERJ Open Res. 2015;45(5):1446–1462. doi:10.1183/23120541.00081-201
- McDonnell MJ, Jary HR, Perry A, et al. Non cystic fibrosis bronchiectasis: a longitudinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respir Med. 2014;109:716–726.
- Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12:1602–1611.
- Chalmers JD, Smith MP, McHugh BJ, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186(7):657–665.
- Winthrop K, Aksamit TR, Olivier KN, et al. The respiratory microbiology of patients with nontuberculous mycobacteria from the United States bronchiectasis research registry. Am J Respir Crit Care Med. 2013;187:A4541.
- EMBARC presentation at the European Respiratory Society congress 2015. The management of bronchiectasis in Europe [cited 2016 Mar 29]. Available from: https://www.bronchiectasis.eu/ers2015
- Henkle E, Daley CL, Griffith DE, et al. Patterns of pharmacotherapy for non-cystic fibrosis bronchiectasis (abstract). Am J Respir Crit Care Med. 2015;191:A2445.
- TOBI Podhaler package insert 2015 [cited 2015 Mar 9]. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/tobipodhaler.pdf
- Hurst JR, Elborn JS, De Soyza A. COPD–bronchiectasis overlap syndrome. Eur Respir J. 2015;45(2):310–313.