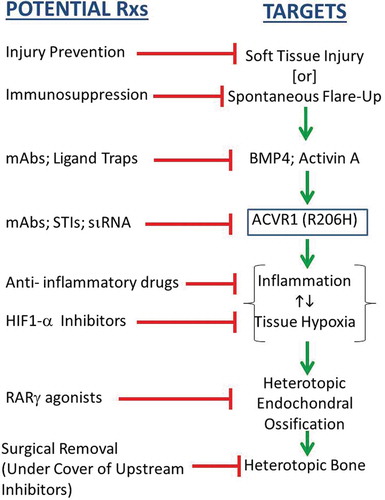
The mutation that causes fibrodysplasia ossificans progressiva (FOP) creates a second skeleton of heterotopic bone for which there is no effective prevention or treatment. Clinical and basic research has unveiled hard targets for therapeutic development.
1. Brief background of FOP
FOP (MIM#135100), a disabling disorder of progressive heterotopic endochondral ossification (HEO), is caused by heterozygous gain-of-function mutations in Activin receptor A, type I (ACVR1, also known as ALK2), a bone morphogenetic protein (BMP) type I receptor. FOP-causing mutations confer a loss of autoinhibition of Smad1/5/8 signaling and altered responsiveness to canonical (BMP) and noncanonical (Activin A) ligands. FOP progresses episodically in trauma-induced or spontaneous flare-ups or insidiously without flare-ups [Citation1–Citation5].
FOP affects approximately one in two million individuals worldwide across all races. Most cases are sporadic, but autosomal dominant transmission with complete penetrance and variable expression are established. FOP severity differs greatly among individuals, even in identical twins (reviewed in [Citation6]).
Malformations of the great toe are present at birth. Flare-ups leading to HEO begin during the first decade of life and appear spontaneously or after muscle fatigue, minor trauma, intramuscular immunizations, or influenza-like viral illnesses. Aponeuroses, fascia, tendons, ligaments, and connective tissue of voluntary muscles are affected. Although some lesions regress spontaneously, most mature by an endochondral pathway to form mature HEO. Except for those following trauma, flare-ups are unpredictable. Disability is permanent. No reliable disease or stage-specific biomarkers have been identified (reviewed in [Citation1,Citation2,Citation6]). Management is currently supportive. High-dose glucocorticoids have been used in acute inflammatory flare ups, with limited efficacy [Citation6].
FOP’s rarity, variability, and fluctuating clinical course pose substantial uncertainties when evaluating experimental therapies [Citation1]. Knock-in mouse models recapitulate the disease phenotype and are vital in testing plausible therapeutic agents [Citation7].
2. Targets and strategies for the prevention and treatment of FOP
A critical unmet need exists for definitive therapies for FOP. The discovery of the causative FOP gene and emerging insights into its mechanisms of action reveal four treatment strategies.
2.1. Strategy 1: blocking activity of the mutant FOP receptor (mtACVR1)
Five approaches are being pursued: signal transduction inhibitors (STIs), blocking monoclonal antibodies against ACVR1, blocking monoclonal antibodies against Activin A, ligand traps, and mutation allele-specific inhibitory RNA.
2.1.1. Signal transduction inhibitors
STIs are important molecular tools for studying BMP signaling in FOP and have great potential for development into powerful therapeutic drugs for FOP [Citation8]. Selective STIs for FOP will inhibit ACVR1 rather than ALK1, ALK3, or ALK6 and are being developed [Citation9]. Broad-spectrum STIs that target ALK2 are also being considered for repurposing in clinical trials.
2.1.2. Blocking antibodies against ACVR1
Mutant ACVR1 demonstrates leaky Smad1/5/8 signaling and ligand hyperresponsiveness, providing a rationale for using blocking antibodies to ACVR1 in the prevention and treatment of FOP. Therapeutic monoclonal antibodies specific for ACVR1 are under development [Citation1].
2.1.3. Blocking antibodies against Activin A
Activin A potently stimulates Smad1/5/8 signaling from mtACVR1 but not from wild-type (wt) ACVR1 [Citation4,Citation5]. Activin A induces HEO in mice expressing mtACVR1 but not in mice expressing only wtACVR1. Inhibition of Activin A with a fully humanized monoclonal antibody blocks spontaneous and trauma-induced HEO in a conditional knock-in model of classic FOP [Citation4]. Activin A is a powerful cytokine that acts as a key regulator of the immune system (reviewed in [Citation10]). Selective activation of mtACVR1 by Activin A highlights an intriguing link between inflammation and HEO in FOP [Citation4,Citation10].
The molecular, physiologic, and structural basis for the sensitivity of mtACVR1 to Activin A is unknown. The unexpected discovery of Activin A in the pathogenesis of FOP identifies a therapeutic target for FOP and excavates a foundation for clinical development.
2.1.4. Ligand traps
Ligand traps have been proposed as a therapeutic strategy in FOP. A soluble recombinant ACVR1-Fc fusion protein (the extracellular domain of human wtACVR1 and the Fc portion of human immunoglobulin gamma 1) abrogates dysregulated BMP signaling caused by mtACVR1 and suppresses chondro-osseous differentiation in vitro [Citation11].
2.1.5. Mutation allele-specific inhibitory RNA
Inhibitory RNA duplexes capable of suppressing the expression of mtACVR1 in connective tissue progenitor cells from FOP patients restores dysregulated BMP signaling to levels observed in control cells and blocks chondro-osseous differentiation (reviewed in [Citation12]). While providing proof-of-principle for allele-specific inhibition of ACVR1 in the prevention of HEO in FOP, the in vivo utility of this approach must be confirmed in mouse models of FOP. A hurdle to human application is safe, effective, and durable delivery of small RNA duplexes to relevant progenitor cells [Citation12]. While small-molecule inhibitors or biologics may dominate near-term therapeutic options, opportunities on the distant horizon using targeted oligonucleotides are appreciable.
2.2. Strategy 2: blocking inflammatory triggers
Clinical findings and mouse models of FOP provide strong evidence of a role for the immune system in triggering and amplifying FOP flare-ups and HEO in the setting of dysregulated BMP signaling (reviewed in [Citation10]). Targeted ablation of mast cells, macrophages, and neuro-inflammatory pathways impairs HEO in mouse models [Citation10,Citation13]. A child with FOP and aplastic anemia (AA) underwent bone marrow transplantation which cured the AA but not the FOP. Subsequent graft-versus-host disease prompted a 15-year course of immunosuppression – during which time the FOP was quiescent. When immunosuppression was discontinued, flare-ups returned [Citation14].
2.3. Strategy 3: blocking responding connective tissue progenitor cells
Activation of the retinoid signaling pathway inhibits chondrogenesis and HEO. Retinoic acid receptor-gamma (RARγ) agonists potently downregulate BMP signaling in pre-chondrogenic cells by promoting the degradation of BMP-pathway-specific Smads [Citation15]. The RARγ agonist palovarotene blocks trauma-induced and spontaneous HEO in a conditional FOP knock-in mouse model [Citation15,Citation16] and is being used in the FDA-approved clinical trials for FOP. Information can be found at: http//:clinicaltrials.gov.
2.4. Strategy 4: blocking the physiologic response to microenvironmental factors that promote heterotopic ossification
Generation of a hypoxic and inflammatory microenvironment in skeletal muscle is a critical step in the formation of HEO [Citation17,Citation18]. Hypoxia-inducible factor 1-alpha (HIF1-alpha) integrates the cellular response to both hypoxia and inflammation and amplifies ligand-independent Smad1/5/8 signaling in the presence of mtACVR1 [Citation18]. Blocking HIF1-alpha pharmacologically with PX-478, apigenin, imatinib, or rapamycin abrogates HEO in FOP mouse models [Citation17,Citation18].
3. Expert opinion
Worldwide interest in FOP research skyrocketed in 2006 following the discovery of the FOP gene. Academia and the pharmaceutical and biotechnology industries have expressed keen interest in FOP and are engaged in research and development to create effective treatments and a cure for FOP.
Successful therapies for FOP will be based on blocking key genetic, molecular, cellular, and tissue targets (). Comprehensive knowledge of the natural history of flare-ups and progressive disability in FOP is of paramount importance in the design of clinical trials. While robust cross-sectional natural history studies have been conducted, knowledge of the longitudinal natural history of FOP is still sparse. An annotated natural history and biomarker study has currently enrolled more than 100 patients and will follow them for over 3 years. Information can be found at: http//:clinicaltrials.gov.
There are several plausible scenarios for clinical trials in FOP: short-term treatment of acute flare-ups, long-term prevention of acute flare-ups, a combinatorial approach, and surgical liberation of ankylosed joints. Different medications and strategies may lend themselves to different clinical trial designs. For example (and in contrast to preclinical studies in FOP), the events around the onset of spontaneous flare-ups in humans are unknown. By the time a patient recognizes a flare-up, disease activity might have been smoldering for days, weeks, or even months. Thus, it is difficult to ascertain the stage of a flare-up that a patient is in or if a drug of interest would be effective at that stage. In contrast, a drug targeted to prevent acute flare-ups would require an acceptable long-term safety profile since the onset of flare-ups is unpredictable and thus preventative treatment would be chronic and lifelong. This is a high hurdle for a kinase inhibitor targeted to block a highly conserved signaling pathway whose blockade may unmask unanticipated side effects. Thus, therapeutic approaches might consider partial blockade of a signaling pathway with a rescue approach targeted for breakthrough flare-ups, should they occur. Finally, due to the tremendous risk to FOP patients of stimulating more extensive HEO and resulting consequences, surgical liberation of ankylosed joints should not be undertaken until proven treatment options are established.
The main goal for FOP treatment is prevention of progressive postnatal HEO. Thus, the battleground for FOP is childhood. Recent identification of agents such as imatinib and rapamycin that target inflammation and hypoxia-sensing pathways might be repurposed compassionately or formally evaluated by clinical trials in children, while novel therapeutics are being developed. STIs currently in non-FOP-related clinical trials that also target ALK2 might be repurposed for early entry into FOP clinical trials.
Importantly, several adults have been identified with the classic FOP mutation and congenital features of FOP but a paucity of postnatal HEO. These resilient individuals hold the key to understanding factors that trigger FOP flare-ups and amplify progression of the disease. Robust investigation is being conducted to decipher the genetic, epigenetic, environmental, and immunologic factors involved. If distinct factors can be identified in these few individuals, new robust targets for therapy are likely to emerge.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgments
The authors thank Mr. Robert Caron and Mrs. Kamlesh Rai for their invaluable technical and administrative assistance, and the present and past members of our research program for their work leading to our current understanding and perspectives on FOP.
Additional information
Funding
References
- Kaplan FS, Pignolo RJ, Shore EM. From mysteries to medicines: drug development for fibrodysplasia ossificans progressive. Expert Opin Orphan Drugs. 2013;1:637–649.
- Pignolo RJ, Bedford-Gay C, Liljesthrom M, et al. The natural history of flare-ups in fibrodysplasia ossificans progressiva: a comprehensive global assessment. J Bone Miner Res. 2016;31:650–656.
- Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2016;38:525–527.
- Hatsell SJ, Idone V, Wolken DM, et al. ACVR1 (R206H) receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to Activin A. Sci Transl Med. 2015;7(303):ra137.
- Hino K, Ikeya M, Horigome K, et al. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci USA. 2015;112:15438–15443.
- Kaplan FS, Shore EM, Pignolo RJ, eds; The International Clinical Consortium on FOP. The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Clin Proc Intl Clin Consort FOP. 2011;4:1–100. Available from: www.ifopa.org
- Chakkalakal SA, Zhang D, Culbert AL, et al. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27:1746–1756.
- Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409–418.
- Dey D, Bagarova J, Hatsell SJ, et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med. 2016;8(366):366ra163.
- Kaplan FS, Pignolo RJ, Shore EM. Granting immunity to FOP and catching heterotopic ossification in the Act. Semin Cell Dev Biol. 2016;49:30–36.
- Pang J, Zuo Y, Chen Y, et al. ACVR1-Fc suppresses BMP signaling and chondro-osseous differentiation in an in vitro model of fibrodysplasia ossificans progressiva. Bone. 2016;92:29–36.
- Lowery JW, Rosen V. Allele-specific RNA interference in FOP: silencing the FOP gene. Gene Ther. 2012;19:701–702.
- Kan L, Lounev VY, Pignolo RJ, et al. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112:2759–2772.
- Kaplan FS, Glaser DL, Shore EM, et al. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89:347–357.
- Shimono K, Tung WE, Macolino C, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med. 2011;17:454–460.
- Chakkalakal SA, Uchibe K, Convente MR, et al. Palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1 (R206H) fibrodysplasia ossificans progressiva (FOP) mutation. J Bone Miner Res. 2016;31:1666–1675.
- Wang H, Lindborg C, Lounev V, et al. Cellular hypoxia promotes heterotopic ossification by amplifying BMP signaling. J Bone Miner Res. 2016;31:1652–1665.
- Agarwal S, Loder S, Brownley C, et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci USA. 2016;113:E338–347.