ABSTRACT
Introduction: Standardization is important across the life cycle of medicinal products, supporting the diagnosis, treatment, and prevention of a wide range of diseases. For rare diseases, standardization is even more important, as patient groups are small, presenting significant challenges in the design, conduct, analysis, and interpretation of clinical studies. It is here that standardization institutions, including the UK’s National Institute for Biological Standards and Control (NIBSC), can have a key role.
Areas covered: A considerable proportion of NIBSC’s work supports the better understanding, diagnosis, treatment, and prevention of rare diseases. NIBSC is also part of the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), creating an agency that is uniquely placed to combine scientific and regulatory expertize for the benefit of public health. This review provides an overview of NIBSC’s work in rare diseases and highlights the positive impact of the work of standardization institutions in this field.
Expert opinion: Standardization in product development is key for patients with rare diseases. The work of standardization institutions is increasingly being recognized as crucial for supporting scientific and clinical advancements, and early and collaborative interactions can provide drug developers with the necessary expertize, when standards matter most.
1. Introduction
In the 1920s, a major challenge in the treatment of diabetes was how to compare the amount of the active substance insulin present in different batches of partially purified pancreas homogenate. Measurement is critical, as too much or too little insulin can cause coma and death of diabetic patients. Pharmaceutical insulin is now quantified by mass spectrometry, but for many biological medicines or ‘biologics’, measuring and assigning a biological activity to these often-complex materials remains a challenging task [Citation1,Citation2]
The National Institute for Biological Standards and Control (NIBSC) was established in the UK in 1972, to formalize, as statutory functions, activities previously overseen by the Medical Research Council [Citation3]. NIBSC merged with the UK’s Medicines and Healthcare products Regulatory Agency [Citation4] in 2013 in order to formalize our long-standing interactions and combine scientific and regulatory expertize for the benefit of public health. Despite its name, the work of NIBSC is not just of national importance but rather has global relevance, ensuring the safety and quality of biologics, including therapeutic products, diagnostics and vaccines, worldwide. Biologics include monoclonal antibodies, clotting factors, immunoglobulins, hormones and growth factors. Biological materials that, due to their complexity, cannot be measured by the same physicochemical methods used for other drugs (e.g. small molecules), and thus require assessment via a bioassay.
NIBSC scientists formulate both written and physical standards – reference materials that can be used to quantify the amount of a biological substance in a given sample. In addition, they perform independent control testing to assure the safety and potency of medicinal products, and perform research to enable and enhance these activities. Biological standards have myriad of applications – positive controls in biological assays (e.g. nucleic acid-based tests and antibody assays), harmonization of data across assays, and are of value in diagnostic, therapeutic, and vaccine development [Citation5]. NIBSC also provides scientific advice to others in the field, including manufacturers of biologics, academics and governmental bodies, and works closely with the World Health Organization’s Expert Committee on Biological Standardization (ECBS), as well as with other international partners. These collaborative activities have led to the development of over 90% of all WHO International Standards (IS) and help to define the international unit of biologics globally.
The availability of materials to help harmonize data, standardize assays, and develop medicinal products is of obvious value. For rare diseases, this is even more important, as patient groups are often small (for example, for orphan designation, rare diseases are defined as conditions not affecting more than 5 in 10,000 people in Europe), presenting a significant challenge in the design, conduct, analysis, and interpretation of clinical studies [Citation6]
A considerable proportion of NIBSC’s work supports the understanding, diagnosis, and treatment of a wide range of rare diseases (see and ). The reasons for this are varied: the availability of effective vaccines might mean that once common diseases are now extremely infrequent; diseases that are rare in Europe and North America remain major health issues in other regions; therapeutics for rare diseases are often biologics and thus attract the interest of a biologics standards Institute.
Figure 1. Contribution that standardization institutions can make to the care of patients with rare diseases.
Standardization institutions contribute to the accurate diagnosis, and appropriate and safe treatment of patients with rare diseases, as well as to the prevention of these diseases. This is possible by the provision of reference materials and assays, performing independent control testing to assure the safety and potency of medicinal products, and undertaking research to enable these activities.
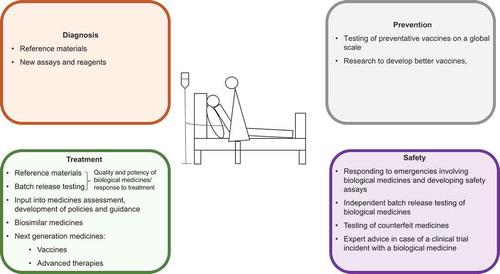
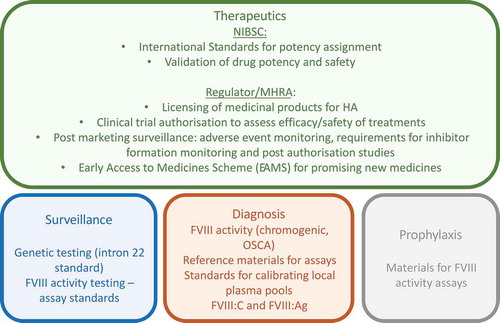
Figure 2. Contribution that NIBSC and the MHRA can make to the care of a patient with Hemophilia A (HA).
This schematic shows the potential impact of NIBSC’s and the MHRA’s work on the treatment, surveillance, diagnosis and prophylaxis of a patient with a rare disease, using HA as an example.
NIBSC is part of the MHRA and together we combine our scientific and regulatory expertize for the benefit of public health. Medicine regulators and standardization institutions have complementary roles, and areas of synergy can be clearly identified. These include the provision of scientific and regulatory expertize, having the ability to influence the development of science-led regulation and innovation, and having an integrated approach to pharmacovigilance (e.g. through establishing complete profiles of antigens in batches of vaccines). NIBSC and MHRA also work closely to investigate and manage risks arising from medicines used in rare diseases (e.g. testing of the quality of licensed or investigational products when serious adverse drug reactions are observed or testing of counterfeit seized mAbs). Medicine regulators’ Innovation Offices provide regulatory advice and guidance, and in the UK expertize from both the medicines regulator (MHRA) and standardization institute (NIBSC) can be requested simulanteously. In addition, in the UK the Regulatory Advice Service for Regenerative Medicines (RASRM), provides a one Stop Shop for research and development professionals, offering a single point of access to joined-up regulatory information, advice and guidance from the Health Research Authority (HRA), the Human Fertilization and Embryology Authority (HFEA), the Human Tissue Authority (HTA), and National Institute for Health and Care Excellence (NICE), and specialists across MHRA, Clinical Practice Research Datalink (CPRD) and NIBSC.
This review provides an overview of the work at NIBSC with an emphasis on rare diseases and highlights the importance of standardization institutions, and the positive impact of their work in this field. Broad areas of diagnosis and surveillance, therapeutics, and prophylaxis will be discussed, and examplars of NIBSC contribution to infectious and genetic diseases will be provided.
2. Diagnostics and surveillance
The low frequency of rare diseases can result in delays and challenges in diagnosis, and the study of low prevalence conditions often requires the collection of data across geographically diverse populations. A patient, whether or not their disease is rare, requires first and foremost, an accurate and timely diagnosis, so that their condition can be managed appropriately. The time from first symptoms to formal diagnosis can be prolonged for patients with a rare disease, as there may be a lack of awareness of the condition due to its scarcity. Variability between diagnostic tests, in terms of their specificity, sensitivity, and accuracy, can be a major challenge, as can the variation between results obtained using the same test but in different laboratories; standardization is therefore critical. Through research and the development of standards, NIBSC contributes to the development and implementation of diagnostic kits, assays, and secondary standards to harmonize and improve the accuracy of diagnostic testing.
Mass and molarity are not appropriate for assigning potency to biological medicines due to their complexity [Citation1,Citation2]. Biological assays are therefore used to assign a unit of measurement, which is specific to the material being evaluated. Primary standards, such as the WHO IS that NIBSC produce, typically have an International Units (IU) assignment and are used to calibrate and ensure consistent, accurate reporting within labs and between labs, especially critical for quantitative diagnostic assays. These standards are also valuable in non-quantitative assays, since sensitivity and reproducibility can be determined.
2.1. Enterovirus environmental surveillance of poliovirus and other enteroviruses
The global polio eradication initiative (GPEI), launched in 1988, is one of the most ambitious and complex public health programs ever attempted [Citation7]. Although complete eradication was not achieved by the year 2000 as projected, the GPEI has been successful in reducing the number of cases by more than 99% since it was launched [Citation8]. Challenges remain in Pakistan and Afghanistan where wild poliovirus is still circulating and in some areas of Africa and Asia where outbreaks due to vaccine-derived poliovirus (VDPV) are ongoing [Citation9]. The genetic instability of the live-attenuated oral polio vaccine (OPV) used in the program means that VDPVs can arise and transmit in areas of poor immunity causing outbreaks and can replicate for long periods of time in immunodeficient patients resulting in the release of virulent viruses into the environment [Citation10]. The longest known VDPV excreter who has been excreting type 2 poliovirus for more than 30 years was described by our polio laboratory at NIBSC [Citation11].
Due to the success of the GPEI in interrupting poliovirus circulation in most regions, poliomyelitis has become a very rare disease in many countries. This has brought new challenges in terms of maintaining capacity for immunization, clinical diagnosis, and surveillance systems to ensure the polio-free status can be sustained and demonstrated. Indeed, the endgame of the GPEI requires new improved approaches in terms of immunization and surveillance systems, as well as the containment of poliovirus serotypes as they become eradicated. As a WHO Collaborating Center for Polio Reference and Research, NIBSC has played a key role in the GPEI since its launch. This contribution helps to ensure the high quality of polio vaccines used in the programme through the establishment of standards and batch release testing, as well as to the development of a global laboratory network to support efficient and sensitive surveillance systems that help monitoring poliovirus circulation, which is essential for designing optimal public health interventions to progress eradication.
The main challenge for the endgame will be to establish rapid direct detection of molecular methods for poliovirus to replace current systems requiring long and complex cell culture algorithms. In addition, there will be a need to expand environmental surveillance that has been shown to be very sensitive in detecting poliovirus circulation in the population even in the absence of paralytic cases, as we have shown in Israel in collaborative research led by the Israel National Polio Laboratory in Tel Aviv [Citation12,Citation13]. NIBSC as part of the WHO Small Working Group for polio diagnosis and in collaboration with Imperial College in London (UK), supported by the Bill and Melinda Gates Foundation (BMGF), is actively pursuing these aims. We have recently developed methods that can detect and identify not only poliovirus but also human enteroviruses directly from cell cultures, stool extracts and sewage concentrates using novel Next Generation Sequencing (NGS) approaches [Citation11,Citation14–Citation19]. This allows us to identify multiple enterovirus serotypes in sewage samples and hence in the human population, including some of public health relevance such as poliovirus, EV-D68, and EV-A71, as well as novel serotypes with potential to cause outbreaks such as EV-C104, EV-C105 and EV-C109.
Using our newly established methods, NIBSC have recently reported an upsurge of EV-D68 detection in wastewater samples from the UK between August and October 2018 [Citation20] in agreement with recent reports of increased EV-D68 detection and the likely association of EV-D68 infection with polio-like cases of acute flaccid myelitis in the UK [Citation21], following similar reports in the USA [Citation22]. Similarly, we have recently identified a new EV-A71 genogroup circulating endemically in Pakistan showing high prevalence and genetic diversity following the analysis of sewage samples collected throughout the country [Citation23].
2.2. Emerging virus diagnosis
Emerging infectious diseases often lack validated diagnostic kits that would allow for an expedited diagnosis and rapid containment of epidemics. In outbreak situations, such as for Ebola, Lassa, Nipah and others, it is vital that infected individuals are promptly identified and appropriately treated and cared for [Citation24]. The Emerging Viruses group at NIBSC has experience in producing reference materials and WHO International Standards for high containment viruses, which require specialized and high-cost containment laboratory facilities. NIBSC has developed procedures to enable the safe production and distribution of International Standards that precludes the need for high containment laboratories. This was achieved through the use of lentiviral vectors to package the nucleic acid of the target genes for nucleic acid amplification-based technology (NAT) assays [Citation25]. This is a completely synthetic approach and avoids the need for handling and culture of wild type viruses. The process is relatively quick (less than 3 months after the viral sequences are available) and therefore allows for the production of reference materials that are contemporaneous to the outbreak [Citation26]. The distribution of these materials to diagnostic labs in the outbreak areas sidestep biosafety issues that would otherwise trigger the need for international permits. NIBSC has successfully produced WHO reference reagents for Ebola virus [Citation27] and CE marked (Conformité Européenne, European Conformatity) working reagent for Zika virus [Citation28] and is currently working on standards for Lassa, Marburg, Sudan, Nipah, MERS, and Crimean-Congo hemorrhagic fever viruses. These rare diseases have high morbidity and case fatality rates, with the potential of epidemics, and have been identified in the priority list of several organizations including the WHO, the UK Vaccine Network (UKVN) and the Coalition for Epidemic Preparedness Innovations (CEPI).
2.3. Hemophilia A (HA) diagnosis
Congenital hemophilia A is a rare sex-linked bleeding disorder affecting around 1 in 5000 males. The extent of the Factor VIII (FVIII) deficit dictates disease severity; patients with endogenous FVIII activity <1% of normal have severe hemophilia A, moderate hemophilia A levels are between 1% and 5%, and mild hemophilia A is ~5–40% of normal.
In order to assess FVIII activity, clinical labs will use either a chromogenic assay (indirect assessment of FVIII activity via the generation of a chromogenic product by activated FXa, which is a FVIII-dependent process) or the one-stage clotting assay (OSCA) (see ), which can be measured as a decrease in the mobility of an object through clot material, or by light absorption. Laboratories will compare FXa activity (chromogenic assay) or clotting time (OSCA) in their patient (‘test’) sample and look for any decrease in FVIII activity compared to the healthy control (100%). The activity of FVIII in the local plasma pool should be calibrated to a reference material to ensure that it is a valid control and can accurately quantify FVIII activity. The specific FVIII activity of the local plasma pool can be determined by comparing it to a reference material with defined FVIII activity (‘potency’). It is recommended by kit manufacturers that a standard curve is carried out for each kit using normal human plasma that has been calibrated to the IS. It is important to regularly check the activity of the local pool to ensure there is no decline in activity over time that would adversely affect the FVIII activity assessment, and to account for population variation in FVIII activity. Clinical labs will also often carry out routine maintenance and checks on their blood analyzers to ensure data are reliable and reproducible. Again, this is often done using an in-house reference material whose potency assignment is traceable back to the IS, produced by the NIBSC. Alternatively, labs can use reference reagents from manufacturers, often supplied by the company that supplies the hospital with the blood analyzers it uses. Ideally, the activity of this reference reagent is traceable to an official standard, but this is not always the case.
Most hemophilia A patients are treated with exogenous FVIII with the aim of keeping their FVIII levels above 1% of normal [Citation29]. The level of circulating FVIII activity guides the dosing regimen of replacement FVIII products (for NIBSC contribution to product testing see the following section), whose turnover and clearance in vivo can vary from patient to patient and must be closely monitored. A serious and fairly common (~30%) complication for patients with severe hemophilia treated with FVIII replacement therapy is the development of antibodies that neutralize FVIII activity (often referred to as ‘inhibitors’) [Citation30,Citation31]. It is vital that this is diagnosed as soon as possible so that appropriate action can be taken to rectify FVIII levels in these patients. Antibody neutralizing activity is determined by the Bethesda assay, a clinical assay in which plasma from a suspected ‘inhibitor’ patient is incubated with normal plasma [Citation32]. If the activity in the normal plasma (assigned unit activity based on calibration with an FVIII standard traceable back to the WHO International Standard 07/350) decreases following the addition of the patient plasma compared to the control, this indicates the presence of an FVIII-neutralizing antibody. While this assay is able to identify the presence of FVIII-neutralizing antibodies, it is notoriously error prone and lacks sensitivity [Citation33,Citation34]. The availability of an FVIII-neutralizing antibody reference material would help in this regard and NIBSC is currently in the process of developing one.
2.4. Human genomics
For more than a decade, NIBSC has been establishing reference materials for human genomic diagnostics, initiated with the WHO international standards for genomic variants associated with Factor V Leiden thrombophilia, Hemophilia A (Factor VIII intron 22 inversion), and Prothrombin (Factor II G20210A), all rare inherited hematological disorders. Such materials allow for the verification of a diagnostic assay’s ability to determine the presence or absence of a genetic marker, and for the patient to have an accurate diagnosis for treatment or health monitoring determination. Similar standards have since been developed for other disease areas, including Fragile X, Prader Willi, and Angelman syndromes. Although effective treatments may not yet be available for such disorders, genetic diagnosis allows for earlier clinical intervention, clinical research and drug development, and the possibility for prenatal screening for subsequent pregnancies for the parents of an affected child. Naturally, there are many more inherited disorders for which genetic diagnosis is usually performed, and thus the scope for further specific reference materials remains. So far, NIBSC has utilized patient-derived cell lines, principally lymphoblastoids, to generate (usually genomic DNA) reference materials. Sourcing such materials is challenging, especially for rare diseases, due to the scarcity of patients and the ethical challenges of obtaining appropriate consent to use the donation for the establishment of reference materials for subsequent distribution to third parties. Biobanks, such as the European Collection of Cell Cultures (ECACC), are a useful source of cells or tissues from rare disease patients, and NIBSC works collaboratively to ensure mutual commercial and scientific benefit with such organizations in the research and development of reference materials.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) genome engineering enables the generation of cell lines containing any genomic variant, negating the need to access rare patient samples, and widens the potential for future reference materials. Meanwhile, NGS approaches are facilitating easier linking of phenotype to genotype for rare genetic diseases, as evidenced by the Genomics England 100,000 genomes project and the routine commissioning of whole genome sequencing for diagnostic purposes across the UK National Health Service [Citation35]. Such exciting approaches will continue to generate volumes of data, which through carefully harmonized diagnostic approaches, and the use of appropriate standards, should provide more patients with an accurate diagnosis.
The detection of cancer genomic markers in the circulatory system gives great potential for the use of ‘liquid biopsy’ in the early detection of disease, with tumors shedding fragmented DNA (circulating tumor DNA; ctDNA), extracellular vesicles, and cells (circulating tumor cells) into the bloodstream [Citation36]. Detection of such markers allows for an early and ongoing, non-invasive, cost-effective approach toward more routine screening for cancer and monitoring of disease recurrence especially in difficult to access tumor sites. However, challenges lie in the harmonization of genomic testing via liquid biopsy, the establishment of concordance between solid tumor and liquid biopsy results, and the establishment of adequate assay sensitivity since these markers are present at very low frequency. An increasing number of molecular assays are available for the detection of ctDNA markers, indicating the speed at which ctDNA-based testing is evolving [Citation37]. NIBSC is currently working to co-develop standards for ctDNA and genomic DNA in its recently established non-invasive biomarkers program, focussing initially on EGFR variants. There is clinical interest in these variants due to their prevalence in non-small cell lung cancer and their association with predicted response to therapeutic tyrosine kinase inhibitors, but this technology is also applicable to rarer cancers [Citation38,Citation39]. Such standards will allow the harmonization of measurement between assays independent of the sample type (liquid or solid tumor biopsy), facilitating the application of ctDNA in clinical practice.
3. Therapeutics and monitoring
All medicines should meet applicable standards of quality, safety, and efficacy. NIBSC’s reference materials have multiple uses in terms of guiding therapeutic decisions: helping to identify the right therapy, evaluating the initial therapeutic response and identifying the development of treatment resistance. The inclusion of reference materials in clinical trial settings also permits calibration and harmonization across assay platforms and between distinct clinical trial sites. In addition to developing reference materials, NIBSC scientists carry out research to improve understanding of disease mechanisms, drug responses, and side effects, supporting the development of new approaches to therapy including advanced therapies, such as cell and gene therapies.
3.1. Biosimilar medicines
Since their introduction in the 1990s, monoclonal antibody (mAb) therapies have revolutionized the treatment of many diseases [Citation40]. Often described as ‘magic bullets’ due to their high selectivity and specificity for their targets, they are generally well tolerated and have found great utility in the treatment of rare diseases with many being granted orphan status in both the USA and Europe, with a recent review identifying 24 already licenced mAbs with orphan status [Citation41]. However, being complex proteins, they are costly to develop and manufacture. Licensed mAbs are now starting to come out of their intellectual property protection periods resulting in intense interest and the development of biosimilar mAb products.
Biosimilar medicines (including mAbs) are biological medicines that are highly similar to another already approved biological medicine (the ‘reference medicine’). Without standardization, these products might vary relative to each other with regards to their critical quality attributes. The WHO recognized this and in collaboration with NIBSC began the development of international potency standards that can be used to harmonize the potency of biosimilar mAbs worldwide. In 2017, NIBSC developed the first WHO IS for rituximab [Citation42], (a mAb targeting CD20 used to treat haematological maligancies but also with an orphan designation in Europe for solid organ transplantation), enabling bioactivity between products to be compared in terms of international units. NIBSC is currently developing other mAb IS, and these monitoring tools will help promote the global harmonization of biosimilar potency contributing to wider patient access.
3.2. Cell and gene therapies
Progress with cell and gene based therapies is rapidly evolving. Gene therapies are designed to edit, correct or replace faulty genes, many of which are associated with rare diseases. Recent examples include Strimvelis (autologous CD34+ enriched cell fraction that contains CD34+ cells transduced with retroviral vector that encodes for the human adenosine deaminase cDNA sequence [ADA] for the treatment of ADA-SCID [severe combined immunodeficiency]), and Luxturna (voretigene neparvovec) for inherited retinal dystrophy. Significant challenges in developing gene therapy for a rare disease arises from its small patient population and often, its occurrence in pediatric patients and the complicated geno- and phenotypes of the disease. Internationally recognized physical standard can be designed to provide a powerful ‘constant-entity’ enabling a direct comparison of cross-assay, cross-trial and cross-patient results. The 1st WHO IS at NIBSC for the integration analysis of lentivirus-based gene therapy is a good example for such purpose [Citation43].
3.3. Human genomics in personalized medicine
Another priority for standardization institutions is the development of standards for cancer genomics, with the presence of a genetic marker linked to the initiation of a specific therapy or personalized medicine. Companion diagnostics are increasingly required to select the appropriate patients for a therapeutic product; many of these are molecular tests for a specific genetic marker. Long-term monitoring of the patient is also usually required, in the evaluation of initial therapeutic response, the emergence of any therapeutic resistance and ultimately remission status. Such testing often requires the quantification of the genetic marker, and thus the availability of standards for consistent, accurate, and sensitive reporting, which should be independent of assay, laboratory, or geography.
By way of example, NIBSC established the WHO 1st International Genetic Reference Panel for the quantification of BCR-ABL1 translocation in 2009 [Citation44]. This has greatly facilitated the global harmonization of BCR-ABL1 measurement of therapeutic response in chronic myeloid leukemia patients, with accurate, sensitive reporting on the international scale, including in aligning commercial diagnostic PCR-based kits, supporting the production of calibrated secondary standards, and underpinning the evaluation and establishment of emerging technologies for BCR-ABL1 diagnostics, such as digital PCR and NGS. Similarly, NIBSC has produced quantitative WHO standards for JAK2 V617F [Citation45], and KRAS codons 12 and 13 mutations [Citation46], and is currently working on standards for cancer genome-wide standards for multiple further genomic markers associated with therapeutic response.
4. Prophylaxis
Prophylaxis can prevent or delay the onset of a disease, but it can also be used as a strategy to minimize disease progress or severity. In disease prevention, NIBSC scientists contribute to the development of safe and effective vaccines in a number of ways: by carrying out independent control testing of vaccines to assess their potency and safety, we help to ensure the delivery of effective vaccines; in addition, NIBSC’s reference materials can be used to support preclinical vaccine development, so that data from non-clinical work determining protective titers can be bridged to clinical trials; we also perform research leading to novel approaches to develop safer vaccines.
4.1. Emerging viruses
NIBSC has developed WHO IS for antibody against Ebola [Citation47] and Zika viruses [Citation48] to calibrate and harmonize assays to measure humoral immune responses and thereby the efficacy or potency for respective vaccine candidates that are currently in development. These typically comprise pools of sera/plasma from convalescent patients and therefore require assurances over viral safety so that they are risk free and have wide utility in a range of laboratory settings. Most of the emerging viruses are classified as high hazard group 3 and 4 and as such need to be handled in relevant high containment laboratories. To overcome this and provide assurance on biosafety, the sera/plasma is treated with solvent/detergent using a validated method. As described in the WHO collaborative study to assess the suitability of an interim standard for antibodies to Ebola, virus inactivation was confirmed by spiking samples with HIV-1 after solvent detergent treatment and demonstration of non-recovery of the virus by subsequent culture on permissive cell lines. This has facilitated our capacity to be able to respond and produce antibody standards for the international community without limiting their use to high containment facilities.
The emerging viruses group has a program of work to produce standards for diseases that are likely to be declared a Public Health Emergency of International Concern (PHEIC) by the WHO and identified through its R&D Blueprint. These include mostly RNA viruses with high case fatality rates, such as Lassa fever, Nipah virus and Middle East Respiratory Syndrome (MERS) coronavirus for which there is an active program funded by the CEPI to produce vaccines for these diseases. Through these activities, we have developed an international presence with links to key stakeholders who can support us in our work to produce standards. We have proposed that a framework [Citation24] is implemented to address the need to produce standards in a timely and efficient manner for an emerging PHEIC.
4.2. Poliovirus and other enteroviruses
NIBSC plays an important role in the batch release of poliovirus vaccines in the UK as well as in their testing for the Prequalification of Medicines Programme (PQP) of the United Nations program managed by WHO. NIBSC is part of a consortium (including the Centers for Disease Control, the University of California, San Fransisco and the US Food and Drug Administration), that is supported by the BMGF and Program for Appropriate Technology in Health (PATH). The consortium is developing new live-attenuated oral PVs (nOPVs) that due to their improved genetic stability will not result in VDPV circulation [Citation49]. Clinical studies using type 2 nOPV candidates are ongoing which will be soon followed by those for type 1 and 3 nOPV products.
Following eradication of wild type 2 poliovirus and the global switch from live trivalent OPV to live bivalent OPV in April 2016, there is a need for universal use of inactivated polio vaccine (IPV) to ensure immune protection against type 2 poliovirus. The WHO, in collaboration with other institutions, including NIBSC, and supported by BMGF and PATH are making concentrated efforts to establish the production of safer IPV based on Sabin OPV strains rather than wild type polioviruses in various countries to make sure the demand for IPV is satisfied. NIBSC has long contributed to research and standardization projects in this area [Citation50–Citation53]. We developed a novel high-resolution identity test for IPV products based on NGS [Citation54] and we have recently led a collaborative study to establish the first International Standard for Sabin-IPV which provides a major contribution to these efforts [Citation55]. Furthermore, hyper-attenuated S19 poliovirus strains unable to replicate in humans have been developed at NIBSC [Citation56] and will be used as seeds for IPV production [Citation57] as well as reagents for essential assays requiring the use of live poliovirus that will now be possible to carry out at lower containment levels, such as neutralization assays for seroprevalence studies or characterization of immunoglobulin preparations for medical use [Citation58]. In addition, NIBSC in collaboration with the Universities of Leeds and Oxford and the John Innes Center (JIC) in the UK has made considerable progress in the research and development of non-infectious vaccine-like particles to be used as vaccines in the post-eradication era [Citation53,Citation59].
NIBSC is leading projects to establish reference standards and methods to help the standardization and quality control of new vaccines and serology studies for emerging non-polio enteroviruses. The first WHO IS for anti-EV71 serum was established in 2017 in collaboration with the National Institute for Food and Drug Control (NIFDC), China [Citation60] and a collaborative study to establish the first IS for EV71 inactivated vaccines is in its advanced stages. Preparations for similar projects for EV-D68 are ongoing.
5. Conclusions
Patients with rare diseases expect high-quality medicines and timely approvals. In order to facilitate this, drug developers can seek scientific advice from medicine regulatory authorities on a wide range of topics, from expertize in manufacturing challenges, to non-clinical requirements, to designing clinical trials, and in particular for driving efficiencies in a product’s development. Standardization agencies, through their contribution to the understanding, diagnosis, treatment, and prevention of rare diseases, support patient access to the right medicines, and accurate diagnosis and monitoring of their disease. In addition, active research links to the discovery of new medicines and therapeutic approaches for future patients, as well as to more accurate and rapid diagnosis. The importance of rare diseases to standardization agencies has been highlighted in this review, clearly demonstrating the public health impact of this work.
6. Expert opinion
Standardization has an indispensable role in rare diseases. The establishment of standards and reference materials, control testing of medicinal products, and research, has a significant global impact on the surveillance, accurate diagnosis, and treatment of a wide range of rare diseases. In this way, standardization agencies around the globe have a clear role, filling a gap in research and medicine evaluation that might otherwise be neglected.
Standardization agencies occupy a unique position at the interface between public health and fundamental research and development, and they need to continuously redefine their remits as science and products develop, and public health needs evolve. In the UK, examples include the expansion of NIBSC standard portfolio for biosimilars, diagnostics, advanced gene, and cell therapies and for environmental surveillance of new and re-emerging viruses. The advancement of standardization will help optimize the development of novel diagnostics and therapeutics for rare disease and will enable comparison of results across studies and across clinical practices, thus providing consistency and confidence in the public health service.
The availability of reference materials and standards harmonizes research and supports research reproducibility and integrity. One area currently not capitalizing on the availability of these reagents is in basic and translational science. The use of these materials allows groups all over the world to standardize reagents from any supplier against a common material; as many drug discovery programs are designed around published observations it is essential that the data is as accurate as possible to avoid wasting time and money, which drives up the cost of medicines and restricts their accessibility and availability. As well as providing materials for the research community as it moves and evolves, we must do the same for biomedicines, providing standards to validate new manufacturing protocols and technologies, new standards for rapid response to emerging health crises, novel therapeutics, new therapeutic areas (microbiome, advanced therapies, and biosimilars), and gene therapies. We encourage discussions with the scientific community to enable us to provide the materials it needs to carry out research to the highest quality and reliability.
Any progress in the field of rare diseases principally depends on collaboration between different specialists, and agencies responsible for biological standardization must also follow this paradigm. Sharing information and collaborating between experts, such as clinicians, veterinarians, and pharmacists in the field, and academic researchers, manufacturers, and regulators in multidisciplinary teams at a national and international level is key. In the UK, NIBSC has a wide network of collaborations both nationally and internationally and along with the MHRA is a partner organization for the planning and delivery of the UK Strategy for Rare Diseases. Industry too can benefit from an enhanced interaction between NIBSC and the regulator, leading to the provision of scientific advice in all aspects of manufacturing, diagnostics, non-clinical and clinical requirements, and solutions to frequently encountered challenges.
The world of rare diseases is far from small and it is expanding. Rare diseases are increasingly being addressed in public health strategies and are attracting the interest of industry and academia in developing novel treatments and diagnostics. Along with the regulator, NIBSC remains committed to screening the rare diseases’ landscape and performing research to proactively set standards in areas of high unmet needs for patients and areas where existing and future technologies, collaborations, and partnerships will allow a significant benefit for patients. For example, greater global access to next-generation sequencing will likely drive the move from single-analyte-based diagnostics to genome-wide analysis, and thus greater genotype-phenotype correlation, especially for rare diseases.
Science, technology, globalization and climate change constantly modify the rare diseases landscape: common diseases can become rare, whilst other diseases re-emerge or new rare diseases appear. Standardization agencies have a duty to keep up-to-date with such changes and to foresee the resultant needs, to ensure that the required standards are available in the future for any possible scenario, and to continue to commonly set the biological standards in rare diseases.
Article highlights
Patients with rare diseases expect high-quality medicines and timely diagnosis and approvals of new medicinal products.
Standardization is important across the life cycle of medicinal products, but especially for products developed for rare diseases.
A considerable proportion of standardization agencies work supports the better understanding, diagnosis, treatment, and prevention of rare diseases.
Standardization agencies around the globe have a clear role, filling a gap in research and medicine evaluation that might otherwise be neglected.
Drug developers can seek scientific advice from medicine regulatory authorities on a wide range of topics, driving efficiencies in medicine development programs.
This box summarizes key points contained in the article.
Declaration of interest
All authors are employees of the MHRA. The views expressed are those of the authors and should not be considered those of the MHRA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ferguson J, Burns C. Yardsticks of science: the origins and future of the international unit. IEEE Instrum Meas Mag. 2017 Dec;20(6).
- World Health Organization. Recommendations for the preparation, characterization and establishment of international and other biological reference standards.Geneva: The Organization; 2006, WHO Technical Report Series, No. 932. 2006. [Cited 2019 Jul 1]. Available from: https://www.who.int/bloodproducts/publications/TRS932Annex2_Inter_biolefstandardsrev2004.pdf?ua=1
- NIBSC. [cited 2019 Jul 1]. Available from: https://www.nibsc.org/
- MHRA. [cited 2019 Jul 1]. Available from: https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency
- Almond N. Toward higher standards in viral diagnostics. The Pathologist. 2017 Feb 24. Available from: https://thepathologist.com/outside-the-lab/toward-higher-standards-in-viral-diagnostics
- O’Connor D, Hemmings R. Coping with small populations of patients in clinical trials. Expert Opin Orphan Drugs. 2014;2(8):765–768. Available from: https://www.tandfonline.com/doi/abs/10.1517/21678707.2014.931221
- Bahl S, Bhatnagar P, Sutter RW, et al. Global polio eradication - way ahead. Indian J Pediatr. 2018;85:124–131. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5775388/
- World Health Organization. Polio case count. [cited 2019 Jul 1]. Available from: https://extranet.who.int/polis/public/CaseCount.aspx
- Zambon M, Martin J. Polio eradication: next steps and future challenges. Euro Surveill. 2018;23(47). DOI:10.2807/1560-7917.ES.2018.23.47.1800625.
- Burns CC, Diop OM, Sutter RW, et al. Vaccine-derived polioviruses. J Infect Dis. 2014;210(Suppl 1):S283–93.
- Dunn G, Klapsa D, Wilton T, et al. Twenty-eight years of poliovirus replication in an immunodeficient individual: impact on the global polio eradication initiative. PLoS Pathog. 2015;11:e1005114.
- Shulman LM, Martin J, Sofer D, et al. Genetic analysis and characterization of wild poliovirus type 1 during sustained transmission in a population with >95% vaccine coverage, Israel 2013. Clin Infect Dis. 2015;60:1057–1064. Epub 2014 Dec 30.
- Shulman LM, Gavrilin E, Jorba J, et al. Molecular epidemiology of silent introduction and sustained transmission of wild poliovirus type 1, Israel, 2013. Euro Surveill. 2014;19:20709.
- Fernandez-Garcia MD, Kebe O, Fall AD, et al. Enterovirus A71 genogroups C and E in children with acute flaccid paralysis, West Africa. Emerg Infect Dis. 2016;22:753–755.
- Fernandez-Garcia MD, Majumdar M, Kebe O, et al. Emergence of vaccine-derived polioviruses during ebola virus disease outbreak, Guinea, 2014-2015. Emerg Infect Dis. 2018;24:65–74.
- Fernandez-Garcia MD, Majumdar M, Kebe O, et al. Identification and whole-genome characterization of a recombinant enterovirus b69 isolated from a patient with acute flaccid paralysis in niger, 2015. Sci Rep. 2018;8:2181.
- Majumdar M, Klapsa D, Wilton T, et al. Isolation of vaccine-like poliovirus strains in sewage samples from the UK. J Infect Dis. 2017. DOI:10.1093/infdis/jix667.
- Majumdar M, Martin J. Detection by Direct Next Generation Sequencing Analysis Of Emerging Enterovirus D68 and C109 strains in an environmental sample from Scotland. Front Microbiol. 2018;9:1956.
- Perepliotchikov Y, Benhar I, Manor Y, et al. A novel magnetic beads-based method for polioviral concentration from environmental samples. J Virol Methods. 2018;260:62–69. Epub 2018 Jul 9.
- Majumdar M, Wilton T, Hajarha Y, et al. Detection of Enterovirus D68 in wastewater samples from the United Kingdom during outbreaks reported globally between 2014 and 2018. IN PRESS.
- Public Health England. PHE investigating rise in reports of rare illness. [ cited 2019 July 1]. Available from: https://www.gov.uk/government/news/phe-investigating-rise-in-reports-of-rare-illness
- McKay SL, Lee AD, Lopez AS, et al. Increase in acute flaccid myelitis - United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:1273–1275.
- Majumdar M, Sharif S, Klapsa D, et al. Environmental surveillance reveals complex enterovirus circulation patterns in human populations. Open Forum Infect Dis. 2018;5:ofy250.
- Rampling T, Page M, Horby P. International biological reference preparations for epidemic infectious diseases. Emerg Infect Dis. 2019 Feb;25(2):205–211.
- Mattiuzzo G, Ashall J, KS D, et al. Development of lentivirus-based reference materials for Ebola virus nucleic acid amplification technology-based assays. PLoS One. 2015 Nov 12;10(11):e0142751.
- Huffner A, Agnandji ST, Combescure C, et al. Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2018 Jul;18(7):738–748. Epub 2018 Apr 5.
- World Health Organization & WHO Expert Committee on Biological Standardization. 2015 Oct 12–16. WHO collaborative study to assess the suitability of interim standards for Ebola virus NAT assays: preliminary report: expert committee on biological standardization: Geneva World Health Organization. [cited 2019 Jul 1]. Available from: http://www.who.int/iris/handle/10665/197763
- Baylis SA, Hanschmann KM, Schnierle BS, et al. Harmonization of nucleic acid testing for Zika virus: development of the 1st World Health Organization International Standard. Transfusion. 2017 Mar;57(3pt2):748–761. Epub 2017 Feb 23.
- Richards M, Williams M, Chalmers E, et al. A United Kingdom Haemophilia Centre Doctors’ Organization guideline approved by the British committee for standards in haematology: guideline on the use of prophylactic factor VIII concentrate in children and adults with severe haemophilia A. Br J Haematol. 2010;149(4):498–507.
- Pratt KP. Anti-drug antibodies: emerging approaches to predict, reduce or reverse biotherapeutic immunogenicity. Antibodies. 2018;7(2):1–18.
- Varthaman A, Lacroix-Desmazes S. Pathogenic immune response to therapeutic factor VIII: exacerbated response or failed induction of tolerance? Haematologica. 2019;104(2):236–244.
- Miller CH, Platt SJ, Rice AS, et al. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012;10(6):1055–1061.
- Raut S, Sands D, Heath AB, et al. Variability in factor VIII concentrate measurement: results from SSC field collaborative studies. J Thromb Haemost. 2003;1(9):1927–1934.
- Verbruggen B, Dardikh M, Polenewen R, et al. The factor VIII inhibitor assays can be standardized: results of a workshop. J Thromb Haemost. 2011;9(10):2003–2008.
- Turnbull C, Scott RH, Thomas E, et al. The 100 000 Genomes Project: bringing whole genome sequencing to the NHS. BMJ. 2018 Published 2018 Apr 24;361. DOI:10.1136/bmj.k1687.
- Lianidou E, Pantel K. Liquid biopsies. Genes Chromosomes Cancer. 2019 Apr;58(4):219–232.
- Deans ZC, Butler R, Cheetham M, et al. IQN path ASBL report from the first European cfDNA consensus meeting: expert opinion on the minimal requirements for clinical ctDNA testing. Virchows Arch. 2019;474:681–689.
- Loong HH, Kwan S-C, Mok T, et al. Therapeutic strategies in EGFR mutant non-small cell lung cancer. Curr Treat Options Oncol. 2018 Sep 29;19(11):58.
- O’Connor D. Editor’s foreword: evolving the rare cancer field. Expert Opin Orphan Drugs. 2018;6(9):507–508.
- Lara V. Marks the lock and key of medicine monoclonal antibodies and the transformation of healthcare. New Haven, USA: Yale University Press. ISBN: 9780300167733].
- Park T, Griggs SK, Suh D-C. Cost effectiveness of monoclonal antibody therapy for rare diseases: a systematic review. BioDrugs. 2015 Aug;29(4):259–274.
- Prior S, Hufton SE, Fox B, et al. International standards for monoclonal antibodies to support pre- and post-marketing product consistency: evaluation of a candidate international standard for the bioactivities of rituximab. MAbs. 2018 Jan;10(1):129–142. Epub 2017 Nov 3.
- Zhao Y, Stepto H and Schneider CK. Development of the first world health organization lentiviral vector standard: toward the production control and standardization of lentivirus-based gene therapy products. Hum Gene Ther Methods. 2017 Aug;28(4):205–214.
- White HE, Matejtschuk P, Rigsby P, et al. Establishment of the first World Health Organization international genetic reference panel for quantitation of BCR-ABL mRNA. Blood. 2010 Nov 25;116(22):e111–7. Epub 2010 Aug 18.
- Sanzone P, et al. Collaborative study to evaluate the proposed WHO 1st International Reference Panel for Genomic JAK2 V617F. World Health Organization & WHO Expert Committee on Biological Standardization. 2016. Collaborative study to evaluate the proposed WHO 1st international reference panel for genomic JAK2 V617F. World Health Organization. [cited 2019 Jul 1]. Available from: http://www.who.int/iris/handle/10665/253054
- Sanzone P Collaborative study to evaluate the proposed WHO 1st international reference panel for genomic KRAS codons 12 and 13 mutations Sanzone, Pia, Hawkins, Ross, Atkinson, Eleanor, Rigsby, Peter, Boyle, Jennifer. et al. 2017 Collaborative study to evaluate the proposed WHO 1st international reference panel for genomic KRAS codons 12 and 13 mutations. World Health Organization. Available from: http://www.who.int/iris/handle/10665/260258
- Wilkinson DE, Hassall M, Mattiuzzo G et al. WHO collaborative study to assess the suitability of the 1st international standard and the 1st international reference panel for antibodies to Ebola virus. 2017 WHO collaborative study to assess the suitability of the 1st international standard and the 1st international reference panel for antibodies to Ebola virus. World Health Organization. [cited 2019 Jul 1]. Available from: http://www.who.int/iris/handle/10665/260257
- WHO. Expert committee on biological standardization. 69th Technical Report Series no. 1016. Geneva: World Health Organisation. 2019
- Macadam AJ, Ferguson G, Stone DM, et al. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. J Virol. 2006;80:8653–8663.
- Martin J, Crossland G, Wood DJ, et al. Characterization of formaldehyde-inactivated poliovirus preparations made from live-attenuated strains. J Gen Virol. 2003;84:1781–1788.
- Westdijk J, Brugmans D, Martin J, et al. Characterization and standardization of Sabin based inactivated polio vaccine: proposal for a new antigen unit for inactivated polio vaccines. Vaccine. 2011;29:3390–3397.
- Wilton T, Dunn G, Eastwood D, et al. Effect of formaldehyde inactivation on poliovirus. J Virol. 2014;88:11955–11964.
- Sanders BP, de Los Rios Oakes I, van Hoek V, et al. Cold-Adapted Viral Attenuation (CAVA): highly temperature sensitive polioviruses as novel vaccine strains for a next generation inactivated poliovirus vaccine. PLoS Pathog. 2016;12:e1005483.
- Mee ET, Minor PD, Martin J. High resolution identity testing of inactivated poliovirus vaccines. Vaccine. 2015;33:3533–3541.
- Crawt L, Atkinson E, Tedcastle A, et al. Differences in antigenic structure of inactivated poliovaccines made from Sabin live-attenuated and wild-type poliovirus strains: impact on vaccine potency assays. J Infect Dis. 2019 Accepted.
- Knowlson S, Burlison J, Giles E, et al. New strains intended for the production of inactivated polio vaccine at low-containment after eradication. PLoS Pathog. 2015;11:e1005316.
- Sanders BP, Oakes Ide L, van Hoek V, et al. Production of high titer attenuated poliovirus strains on the serum-free PER.C6((R)) cell culture platform for the generation of safe and affordable next generation IPV. Vaccine. 2015;33:6611–6616.
- Farcet MR, Modrof J, Rabel PO, et al. Continued use of poliovirus after eradication: hyper-attenuated strains as a safe alternative for release testing of human immunoglobulins. Transfusion. 2018;58(Suppl 3):3084–3089.
- Fox H, Knowlson S, Minor PD, et al. Genetically thermo-stabilised, immunogenic poliovirus empty capsids; a strategy for non-replicating vaccines. PLoS Pathog. 2017;13:e1006117.
- Cooper G, Mao Q, Crawt L, et al. Establishment of the 1st WHO international standard for anti-EV71 serum (Human). Biologicals. 2018;53:39–50.
