Abstract
Purpose: A prehabilitation phase is suggested as the ideal way to prepare patients for optimal outcomes from surgery. Our aim was to describe the lessons we learned from developing PREPARE, an evidence-based prehabilitation programme based on a cognitive behavioural approach designed to improve functional outcomes after lumbar fusion surgery.
Methods: We used the Medical Research Council (MRC) framework approach for developing and designing a complex intervention, specifically the two phases; ‘Development’ and ‘Feasibility and Piloting’. Various aspects of treatment fidelity were evaluated during the phase ‘Feasibility and Piloting’. As part of the feasibility element, a pilot Single Subject Research Design study was performed. Eleven patients awaiting lumbar fusion surgery participated in the study. We evaluated in particular the use and application of outcome measures.
Results: Significant lessons were learned which we used to adjust the prehabilitation programme to better fit a surgical context. The original treatment manual was elaborated on, the outcome measures adjusted, and the content of the intervention altered. Finally, the PREPARE programme was published in the form of a study protocol.
Conclusions: There are significant lessons to be learned from testing a study protocol before it is implemented in a large-scale randomised controlled trial (RCT).
Introduction
From a global perspective, low back pain (LBP) causes more disability than any other condition [Citation1]. In line with this, the number of people who undergo lumbar spine surgery has increased worldwide [Citation2], with spinal fusion surgery being the most costly procedure [Citation3–5]. The major reason for elective lumbar spine surgery is degenerative disease, incorporating mainly disc herniation, spinal stenosis and degenerative disc disease.
The outcome of spinal surgery is often, however, not optimal and researchers have tried to identify predictive factors that might influence the outcome [Citation6, Citation7]. The negative impact of poor baseline functional status, high pain intensity and low quality of life scores is a consistent finding after surgery [Citation4]. Psycho-social factors have been found to influence disability and functional outcomes negatively for up to 2 years after lumbar spine surgery [Citation6]. Psychological factors, such as fear of movement (kinesiophobia) in combination with pain catastrophising have been identified as important mediators of disability, disuse and depression in persons with chronic LBP [Citation8, Citation9]. Kinesiophobia has been shown to be present in 70% of persons with LBP scheduled for surgery [Citation10] and is proven to influence the outcome of lumbar spine surgery negatively [Citation11]. Moreover, higher pre-surgical levels of pain catastrophising significantly predicted greater functional disability and back pain in persons who underwent spinal fusion surgery [Citation7].
It has been proven that by applying cognitive behavioural principles to reduce catastrophising thoughts and fear, the level of function increases and the degree of depressed mood decreases [Citation12]. Rehabilitation programmes have been set up to address these factors postoperatively [Citation13]. The period before surgery is suggested to be the ideal phase to prepare patients if optimal outcomes of surgery are to be promoted [Citation14]: however, programmes to address these factors preoperatively have not been studied so far. Therefore, we wanted to design an intervention to address these issues before surgery, and assess by using a randomised controlled trial design (RCT) whether this would improve surgical outcome. Our planned intervention was intended to be complex addressing all characteristics suggested by Craig et al. [Citation15, Citation16]. These are: number of interacting components within the experimental and control interventions; number and difficulty of behaviours required by those delivering or receiving the intervention; number of groups or organisational levels targeted by the intervention; number and variability of outcomes and degree of flexibility; and determining whether tailoring the intervention is permitted. We, therefore, decided to apply the Medical Research Council (MRC) framework approach for developing a complex intervention [Citation15] in the development of our planned RCT [Citation17].
Aim of the study
The overall aim of this study was to describe the lessons we learned from developing PREPARE – a prehabilitation programme based on a cognitive behavioural approach – for patients with degenerative disc disease, using the MRC framework approach for developing a complex intervention.
Methods
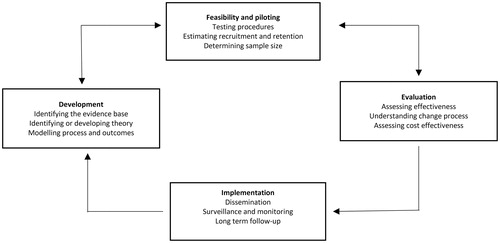
We used the MRC framework for developing and designing this new complex intervention [Citation15]. The process of developing a complex intervention consists of several phases. For the purpose of this study, we describe two of these phases; ‘Development’ and ‘Feasibility and Piloting’. The planned RCT is part of the third phase, ‘Evaluation’ and will be published elsewhere. The phases should, however, not be seen as linear, but as part of an iterative process ().
Figure 1. Key elements of the development and evaluation process as described by the Medical Research Council (MRC) framework for developing a complex intervention.
Medical Research Council Phase 1: development
Who are we interested in?
This project (including all phases below) concerns patients diagnosed with degenerative disc diseases (DDD) by an orthopaedic surgeon and who were on the waiting list for lumbar fusion surgery for one to three segments of the lumbar spine [Citation17].
Identifying the evidence base
First, we reviewed the literature and identified the ‘best evidence based practice’ [Citation18] within the area. At the time of the start of this phase (2012), there was neither any systematic review nor a meta-analysis available within the spinal fusion surgical context. There were, however, several studies available on how to reduce pain-related fear. The best evidence-based practice for reduction of fear and pain catastrophising had at the time been developed by a group of researchers and clinicians in Maastricht, the Netherlands. They had shown that by applying cognitive behavioural principles to reduce pain catastrophising and fear, the degree of depressed mood decreased and the level of function increased [Citation12, Citation19]. There was, however, no knowledge on how to apply these principles in the orthopaedic context before lumbar fusion surgery. Contact was made with this group, and a collaboration established in the form of an ERASMUS exchange programme (Lifelong Learning Programme, Erasmus Code: XXXXXXXXXX).
Identifying or developing theory
Based on modern pain research, treatment strategies have changed from pain reduction to pain management. Focus is nowadays on teaching patients to manage their cognitive (thoughts) and affective (feelings) components of pain in order to optimise functioning in daily life. For patients of working age with LBP, there is evidence that treatment should be multidisciplinary combining cognitive behavioural techniques with physical activity [Citation20]. Fear of movement (affective component of pain) is one factor, frequently studied during the last decade [Citation21]. Fear of movement (kinesiophobia in its’ most extreme form) in combination with pain catastrophising (cognitive component of pain), will according to Vlaeyen’s ‘cognitive-behavioural fear-avoidance model’, lead to avoidance behaviour, subsequently resulting in disability, disuse, depression and social isolation [Citation22]. Based on that theoretical model, Vlaeyen et al. [Citation23] and Linton [Citation24] developed an ‘exposure in vivo’ treatment strategy. During recent years, this treatment was further adapted on the basis of new theoretical insights and scientific evidence from clinical practice [Citation12, Citation25, Citation26].
Modelling process and outcomes
In 2012, three of the authors (AG, HL and ML) visited the pain rehabilitation team at the Maastricht University Medical Centre to discuss and to observe how their version of the exposure in vivo treatment was performed [Citation12, Citation19]. The original Maastricht protocol was an extensive treatment protocol consisting of 14 sessions delivered by a multidisciplinary team [Citation27]. Based on previous successful results from a brief intervention [Citation24, Citation28], we wanted to adapt this programme to be feasible within the orthopaedic context when delivered by a physical therapist pre-operatively. Based on that visit, the authors designed a first draft of Study Protocol version 1.0 for patients awaiting lumbar spine fusion for DDD. This was based on the theoretical model described above [Citation22], and the principles used for cognitive exposure in vivo treatment in previous publications [Citation12, Citation27, Citation29]. Thereafter, an evolving and iterative process followed to adapt this draft protocol into a final version. This process and how the outcomes were evaluated are described below.
Medical Research Council Phase 2: feasibility and piloting
Since complex interventions work best if they are tailored to local circumstances rather than being standardised [Citation15], the feasibility of the Study Protocol version 1.0 applied in the orthopaedic surgical context was investigated in two steps as described below. First, the therapist responsible for the intervention tested parts of the procedure and the use of the outcome measures on a few patients (Step 1). Second, the Study Protocol version 2.0 was tested in the design of a SSRD study (Step 2). This process of continuous assessment, monitoring and enhancement of the reliability and the internal validity of a study is also referred to as treatment fidelity [Citation30]. A starting point to ensure good fidelity is to make sure that the intervention contains treatment elements that are theoretically expected [Citation30]. As described above this intervention rests on a clear theoretical basis. Other aspects of treatment fidelity, such as treatment integrity, treatment receipt and treatment differentiation were also evaluated during the whole process of this study.
Treatment fidelity – testing procedures and training of the therapist – Step 1
In order to facilitate treatment fidelity the working procedure, the therapist’s skills and the use of outcome measures of the Study Protocol version 1.0 were tested on a few patients with DDD waiting for lumbar fusion surgery.
Treatment integrity (whether the treatment was delivered as intended) was investigated by two of the authors from Maastricht (RS and MH) who came to Gothenburg to supervise the delivery of the intervention and to revise the Study Protocol version 1.0. In practical terms, it meant that MH (from Maastricht) observed HL (therapist from Gothenburg) while she provided the intervention. Based on the therapist’s experiences of performing the intervention on the pilot patients, and the observer’s feedback revisions were made in discussion with the research group [Citation27]. Results of these discussions will next be described as well as the evolving revisions resulting in a treatment manual of Study Protocol version 2.0 ().
Another aspect of treatment fidelity is treatment receipt (assessing and optimising the degree to which the participant understands and demonstrates the knowledge provided by the intervention). According to the therapist’s experiences, the pilot patients struggled to complete the Photograph Series of Daily Activities (PHODA) [Citation24, Citation31], in its original format. Specifically, the pilot orthopaedic patients struggled to understand the phrasing of the question ‘How harmful do you think this activity will be to your back?’. According to the therapist, all pilot patients argued that their back were already harmed which was why they had decided to undergo surgery. Based on a discussion in the research group the opening question was, therefore, altered to; ‘How afraid are you of doing the activity the person is doing in the picture?’
Testing procedures and outcomes – Step 2
To assess the feasibility of the Study Protocol version 2.0 and to look into the treatment differentiation (whether the treatment differed in an intended manner) between the pilot intervention and conventional care intervention, a SSRD study was performed. More specifically, we wanted to investigate the following questions:
did the dependent variables that were measured daily (kinesiophobia, catastrophising, physical activity and functioning,) show any changes after the pilot intervention?
did the dependent variables that were measured daily show any changes after conventional care intervention?
could a cognitive shift be detected as shown by the dependent variable catastrophising, measured daily during the pilot intervention or during conventional care intervention?
did a change occur in the dependent outcome measures after the SSRD study?
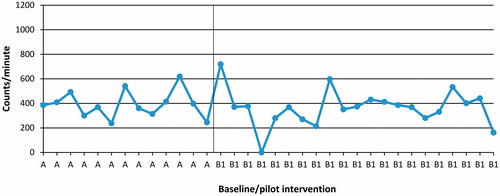
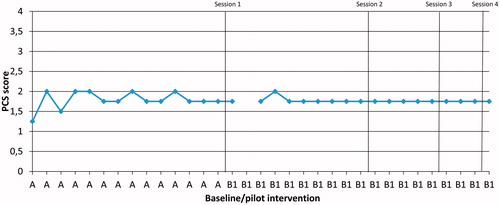
This SSRD study was set up as an A-B-C design [Citation32]. The design uses a baseline phase (A), an intervention phase (B) and a follow-up point (C). During Phase A, the goal was to follow the variability of the dependent variables measured daily (before the interventions start). The B-phase consisted of the pilot intervention (B1: 4 sessions) and the conventional care intervention (B2: 1 session). Phase A was between 8 and 14 d and the intervention phase (B1 and B2) 5–21 d, due to the different number of sessions (B1: 4 sessions and B2: 1 session) and the participant’s availability to take part in the study. All participants received both interventions (B1 and B2) but in a different order. The changes in Phases B1 and B2 as compared to Phase A were analysed visually describing the daily changes in the dependent variables measured daily in level, trend and variability for each patient [Citation33]. Six patients received the pilot intervention B1 first and five patients received B2 first. These changes are illustrated in graphs with delineation of the phases; some examples are presented in . We also evaluated the dependent outcome measures before start of Phase A and at the follow-up Point C in order to identify any changes after the entire study.
Figure 3. Participant P6, daily measures of mean kinesiophobia (TSK) during phase A and phase B1 (pilot intervention).
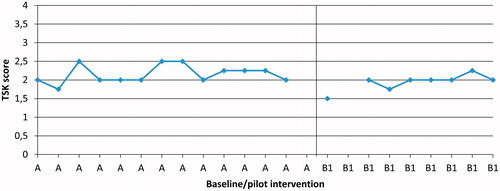
Figure 4. Participant P6, daily measures of mean pain catastrophising (PCS), during Phases A and B1 (pilot intervention).
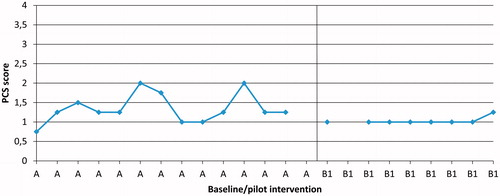
Figure 5. Participant P1, daily measures of three activities, patient specific functional scale (PSFS), during Phases A and B1 (pilot intervention).
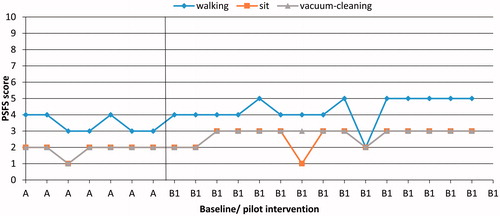
Participants
Seven women and four men were recruited from a private spine clinic in Gothenburg, Sweden from January to November 2013. The sample size of the pilot study was based on the recommendation in the literature for a pilot study [Citation34]. The participants had been diagnosed with degenerative disc disease and were scheduled for lumbar spinal surgery. Participants might have additional minor radiating symptoms with or without simultaneous planned surgical procedure for disc herniation, spinal stenosis, or isthmic spondylolisthesis. Participants were excluded if they had undergone previous decompression surgery for spinal stenosis, or had a spinal malignancy, a confirmed neurological or rheumatic disorder, deformities in the thoracolumbar spine (e.g. idiopathic scoliosis) or a poor understanding of the Swedish language. All participants were invited to participate by HL and all gave written informed consent. The study was approved by the Regional Ethical Review Board in Gothenburg (Dnr. 586-11, Dnr 7527-15).
Procedure of the pilot study
Before the SSRD study started all participants went to the private spine clinic for a base-line evaluation (before Phase A). Demographic data were collected by routine use of the Swedish Spine Register (Swespine) (gender, age, BMI, pain duration and sick-leave) (), one participant responds to the question of comorbidity. To assess the dependent outcome measures the questionnaires kinesiophobia (TSK), pain catastrophising (PCS), back and leg pain (VAS) and disability (ODI) were used and completed before Phase A. At the end of the SSRD study (Point C), the same dependent outcome measures Tampa Scale of Kinesiophobia (TSK), Pain Catastrophising Scale (PCS), visual analogue scale (VAS) (back/leg) and Oswestry Disability Index (ODI) were repeated. Furthermore, during Phase A and B the daily dependent variables were measured by answers to questions regarding kinesiophobia (TSK, four items), pain catastrophising (PCS, four items) and patient-reported functioning (PSFS, three activities). They were also provided with an accelerometer to measure daily physical activity. Participants were instructed to wear the accelerometer every day during Phase A and B and only remove the device while sleeping, swimming or bathing. The evaluation was performed by HL, who also guided the intervention.
Table 1. Demographic description for each patient included in the pilot study.
For all participants (n = 11), Phase A started the day after the base-line evaluation. During Phases A and B a diary with the daily measured dependent variables was sent daily by e-mail to the participants. All participants received the pilot intervention from the same physiotherapist (HL). This physiotherapist had more than 10 years of clinical experience of patients with LBP, and had also 1½ years education and training holding a ‘Graduate Diploma in Cognitive and Behavioural Psychotherapy’.
The conventional care intervention comprised one session of conventional preoperative information for patients awaiting lumbar fusion surgery. The preoperative information included a core exercise programme and details of the post-operative mobilisation routine. Furthermore, the patient was encouraged to stay active and to start performing the recommended exercises before surgery.
It was delivered at the same spine clinic by two physiotherapists not involved in delivering the pilot intervention and with several years of experience with this patient group.
Dependent outcome measures
Kinesiophobia was subjectively rated by the participants using the Swedish version of the TSK. The total score ranges from 17 to 68, with higher scores indicating higher degrees of kinesiophobia. A cut-off score of >37 points was used to indicate kinesiophobia [Citation35]. A minimal important change (MIC) for TSK with a reduction of six points has been reported in a similar population [Citation36]. Pain catastrophising was measured using the Swedish version of the PCS. The total score ranges from 0 to 52, with 0 indicating no pain catastrophising [Citation37]. A cutoff score of 20 points was used to indicate a moderate level of pain catastrophising [Citation38]. A MIC value for PCS with a reduction of 38% from baseline has been reported [Citation39]. Back and leg pain intensity levels over the last week were measured using 100-mm VASs [Citation40]. An intensity score of less than 10 mm was taken as no pain and a score of 20–25 mm was taken as relevant pain [Citation41]. A MIC value for VAS with a reduction of 15 mm for back pain intensity and 17 mm for leg pain intensity has been reported [Citation42]. Disability was measured using the revised version 2.0 of the ODI, Oxford, United Kingdom. Values on the ODI of 21–40 points represent moderate disability; 41–60 points severe disability; 61–80 points incapacitating disability; and 81–100 points restricted to bed [Citation43]. A MIC value for ODI with a reduction of eight points has been reported in similar population [Citation44].
Dependent variables measured daily
For the daily measure of kinesiophobia four items (1, 12, 13 and 16) of the TSK were used [Citation25, Citation35]. For the daily measures of pain catastrophising four items (2, 6, 12 and 13) from the PCS were selected [Citation25, Citation37]. The items were selected based on the literature [Citation23, Citation25] in combination with a discussion in the research group on the likely most sensitive items to detect the changes aimed for by the pilot intervention.
For the daily measure of patient-reported functioning, the Patient-Specific Functional Scale (PSFS) was used [Citation45, Citation46]. In the PSFS, the patient lists three activities that are limited by the condition for which he/she is seeking treatment. The patient then rates his/her perceived difficulty in performing each of the listed activities on a scale from 0 to 100 mm, with 0 indicating that the patient cannot perform the activity at all, and 100 indicating no problem [Citation46].
For the daily measure of physical activity level as mean counts per minutes, a digital triaxial accelerometer was used (ActiGraph GT3X+; ActiGraph, Pensacola, FL). The accelerometer was attached with an elastic band to the participant’s right iliac crest during waking hours.
Results – testing procedures and outcomes
Before the start of Phase A five out of eleven participants presented with kinesiophobia (TSK >37) and seven presented with high levels of catastrophising thoughts (PCS >20). All participants presented with a moderate to high pain intensity level in the back and four had high pain intensity in the leg (NRS >40). All participants suffered from disability and seven of eleven presented with severe disability (ODI >40) ().
Table 2. Descriptive scores for the variables fear of movement, pain catastrophising, pain intensity and disability at baseline and after completion of the pilot study.
Results regarding daily changes in the dependent variables measured daily after the pilot intervention or the conventional care intervention
Following the pilot intervention (B1), kinesiophobia decreased in two participants (P1 and P6) out of six compared to baseline (). Following conventional care intervention (B2) none of five participants showed any changes in kinesiophobia compared to baseline.
Following the pilot intervention (B1) pain catastrophising decreased in three (P1, P4 and P6) out of six participants compared to baseline. An example of such a decrease is shown in 6). Following conventional care intervention (B2), one participant (P9) had a lower level of pain catastrophising compared to baseline.
Following the pilot intervention (B1), one participant out of five (P1) improved in all three important daily activities (to walk, to sit on a chair and vacuum-cleaning) (PSFS) (). One participant (P3) improved in two activities (to walk and vacuum-cleaning) compared to baseline. For one participant (P10) (pilot intervention), the variable PSFS could not be analysed since the daily measures were not completed. Following conventional care intervention (B2), one participant out of five (P9) improved in two activities (to play football with his son and empty the dishwasher) compared to baseline.
Following the pilot intervention (B1), three participants out of six (P1, P3 and P8) became more physically active compared to baseline. However, high individual variation of the physical activity pattern was shown among the participants. One example is shown in . Following conventional care intervention (B2), one participant out of four (P9) became more physically active compared to baseline. For one participant (P2) (conventional care intervention, B2), the variable physical activity level measured daily were lost due to computer problems.
Figure 8. Modified version of the cognitive behavioural fear-avoidance model originally presented by Vlaeyen et a. [Citation49].
![Figure 8. Modified version of the cognitive behavioural fear-avoidance model originally presented by Vlaeyen et a. [Citation49].](/cms/asset/b179bf94-2048-4095-b209-0cbfbf62a665/iejp_a_1553999_f0008_b.jpg)
Results bearing on whether a cognitive shift could be detected in the dependent variable PCS measured daily after pilot or conventional care intervention
Following the pilot intervention (B1), a cognitive shift in the daily-measured PCS could be seen in three participants (P1, P4 and P6) out of six. The changes occurred after the first two sessions (). Following conventional care intervention (B2), a cognitive shift in daily-measured PCS could be seen in one (P9) out of five participants.
Results regarding if a change occurred in the dependent outcome measures after the SSRD study
After the SSRD study, six of eleven participants had a lower score on TSK (kinesiophobia), and two participants reach a MIC (six points reduction) [Citation36]. Five of eleven participants reported a lower score on the PCS (pain catastrophising) and three participants reach a MIC (38% reduction) [Citation39]. Two participants experienced lower back pain intensity (VAS), and two had a lower level of leg pain intensity (VAS) and one participant reach a MIC in back pain intensity (15 mm reduction) [Citation42]. Eight of eleven participants reported a lower score on the ODI (disability) and three participants reach a MIC (eight points reduction) [Citation44] (). We concluded that the dependent outcome measures of interest were sufficient to detect changes after the SSRD study, but the changes were small.
Medical Research Council Phase 3: evaluation and contextual adaptations
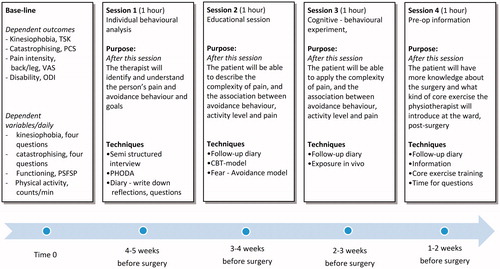
The overarching aim of performing a pilot study was to investigate whether the intervention, as adjusted from the original ‘Maastricht exposure intervention’ [Citation12, Citation27], was feasible to implement in the present context (orthopaedic setting). Based on the results from the SSRD study, we concluded that the intervention did lead to changes reflected in the dependent outcome measures. We also detected small additional changes in the dependent daily outcome variables in those participants receiving the pilot intervention first. Important lessons were learned from delivering the pilot intervention. We concluded that kinesiophobia (TSK) and pain catastrophising (PCS) varied among the participant in the pilot study. Therefore, we decided that we wanted to change the intervention to a more person-centred approach to address these fluctuations. To further strengthen the intervention the content of session 4 was changed to a session of goal setting to be reached four and eight weeks after surgery ( and ). The adjustment of the Study Protocol version 2.0 and the theoretical framework are further presented in and . Possible threats to treatment fidelity were analysed and adjusted and the changes made are presented in .
Figure 9. The PREPARE Study Protocol 3.0 to be tested in an RCT design [Citation17].
![Figure 9. The PREPARE Study Protocol 3.0 to be tested in an RCT design [Citation17].](/cms/asset/b2cd1e15-c0ae-4e6a-815c-aac53b50ad69/iejp_a_1553999_f0009_c.jpg)
Table 3. Changes made after the pilot intervention.
Based on the results of the SSRD study, the contextual adjustments mentioned above were made to the PREPARE programme. Finally, the PREPARE programme was written up in the form of a published study protocol () [Citation17].
Discussion
This study aimed to explain the process of developing a complex intervention and the steps taken along the way to improve the quality of an intervention (PREPARE) to be studied in future RCT [Citation17]. We found that the first pilot intervention, the discussion in the research group and the SSRD study was invaluable in the contextual adaptation of the intervention for a subsequent RCT. Our intervention has been thoroughly planned in several steps. We used the MRC framework for developing a complex intervention to design and develop this new intervention [Citation15]. Although a time-consuming process, it is invaluable to construct the intervention stepwise and the process was essential for to the final outcome. However, by using this thorough design our RCT was delayed in starting time and other researchers should keep this in mind when planning to evaluate a new intervention. Despite this time-consuming process, we are very confident that the intervention delivered and the data from the RCT will as a result be more trustworthy and generalisable to the context of prehabilitation in lumbar spine surgery practice.
High-quality research demands a collaborative and smoothly flowing process. Without good relationships, the implementation of a research project can be jeopardised [Citation47]. We would believe that the time spent on planning this project was a sound investment. We built a solid working process, identifying relevant stakeholders to be the gate keepers in the inclusion process and identifying possible differences at various inclusion sites of the RCT. It is necessary that the people involved in the project understand the purpose of the research. A recent study showed that if people involved in a project did not understand its real aim, and, therefore, had negative expectations, the study would have a poorer outcome [Citation48].
In this study, treatment fidelity was understood as a process applied in the study design, training the provider, delivering of treatment, receipt of treatment and enactment of treatment skills [Citation30]. Possible threats to treatment fidelity were analysed and lessons learned from them lead to adjustment in the final treatment manual [Citation17]. This treatment manual was then to be tested in an RCT study design. Lessons learned from that study will contribute to further insights in terms of treatment fidelity.
Based on what we have learned from this study, we would make some final recommendations. First, we recommend that research peers use a structural framework, such as that of the MRC and plan ahead thoroughly for each step of their complex intervention [Citation15]. Second, we recommend our peers to include a process evaluation, and in particular identify contextual factors a priori and then plan for how to address these, as recommended by Moore et al. [Citation47]. Finally, we recommend our peers to share lessons learned in papers similar to this so that we can learn from each other and build a stronger case together.
| Abbreviations | ||
| BMI | = | Body mass index |
| LBP | = | low back pain |
| DDD | = | degenerative disc diseases |
| MIC | = | minimal important change |
| MRC | = | the Medical Research Council |
| ODI | = | Oswestry Disability Index |
| PCS | = | Pain Catastrophising Scale |
| PHODA | = | Photograph Series of Daily Activities |
| PSFS | = | Pateint-Specific Functional Scale |
| RCT | = | Randomised Controlled Trial |
| SSRD | = | Single Subject Research Design |
| Swespine | = | the Swedish Spine Register |
| TSK | = | Tampa Scale of Kinesiophobia |
| VAS | = | Visuell Analogue Scale |
Disclosure statement
The authors confirm that they have no competing interest to disclose.
Additional information
Funding
References
- Hoy D, March L, Brooks P. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968–974.
- Deyo RA, Mirza SK, Martin BI. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265.
- Mancuso CA, Duculan R, Girardi FP. Healthy physical activity levels below recommended thresholds two years after lumbar spine surgery. Spine (Phila Pa 1976). 2017;42:E241–e247.
- McGirt MJ, Bydon M, Archer KR, et al. An analysis from the Quality Outcomes Database, Part 1. Disability, quality of life, and pain outcomes following lumbar spine surgery: predicting likely individual patient outcomes for shared decision-making. J Neurosurg Spine. 2017;27:357–369.
- Weiss AJ, Elixhauser A, Andrews RM. Characteristics of operating room procedures in U.S. hospitals, 2011: statistical brief #170. Healthcare cost and utilization project (HCUP) statistical briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
- Mannion AF, Elfering A, Staerkle R, et al. Predictors of multidimensional outcome after spinal surgery. Eur Spine J. 2007;16:777–786.
- Abbott AD, Tyni-Lenné R, Hedlund R. Leg pain and psychological variables predict outcome 2–3 years after lumbar fusion surgery. Eur Spine J. 2011;20:1626–1634.
- Vlaeyen JW, Morley S. Cognitive-behavioral treatments for chronic pain: what works for whom? Clin J Pain. 2005;21:1–8.
- Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332.
- Lundberg M, Larsson M, Ostlund H, et al. Kinesiophobia among patients with musculoskeletal pain in primary healthcare. J Rehabil Med. 2006;38:37–43.
- Svensson GL, Lundberg M, Ostgaard HC, et al. High degree of kinesiophobia after lumbar disc herniation surgery. Acta Orthop. 2011;82:732–736.
- Leeuw M, Goossens ME, van Breukelen GJ, et al. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain. 2008;138:192–207.
- Abbott AD, Tyni-Lenné R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine (Phila Pa 1976). 2010;35:848–857.
- Spain J. Prehabilitation. Clin Sports Med. 1985;4:575–585.
- Craig P, Dieppe P, Macintyre S. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655.
- Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to improve health. BMJ . 2000;321:694–696.
- Lotzke H, Jakobsson M, Brisby H, et al. Use of the PREPARE (PREhabilitation, Physical Activity and exeRcisE) program to improve outcomes after lumbar fusion surgery for severe low back pain: a study protocol of a person-centred randomised controlled trial. BMC Musculoskelet Disord. 2016;17:349.
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72.
- Vlaeyen JW, de Jong J, Geilen M, et al. Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behav Res Ther. 2001;39:151–166.
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9:Cd000963.
- Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147.
- Vlaeyen JW, Kole-Snijders AM, Boeren RG, et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372.
- Vlaeyen JW, De Jong JR, Onghena P, et al. Can pain-related fear be reduced? The application of cognitive-behavioural exposure in vivo. Pain Res Manag. 2002;7:144–153.
- Linton SJ, Ryberg M. A cognitive-behavioral group intervention as prevention for persistent neck and back pain in a non-patient population: a randomized controlled trial. Pain. 2001;90:83–90.
- de Jong JR, Vangronsveld K, Peters ML, et al. Reduction of pain-related fear and disability in post-traumatic neck pain: a replicated single-case experimental study of exposure in vivo. J Pain. 2008;9:1123–1134.
- den Hollander M, de Jong JR, Volders S, et al. Fear reduction in patients with chronic pain: a learning theory perspective. Expert Rev Neurother. 2010;10:1733–1745.
- Asmundson GJG, Vlaeyen J, Crombez G. Understanding and treating fear of pain. Oxford: Oxford University Press; 2004.
- Boersma K, Linton S, Overmeer T, et al. Lowering fear-avoidance and enhancing function through exposure in vivo. A multiple baseline study across six patients with back pain. Pain. 2004;108:8–16.
- Vlaeyen JW, de Jong J, Geilen M, et al. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clin J Pain. 2002;18:251–261.
- Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451.
- Leeuw M, Goossens ME, van Breukelen GJ, et al. Measuring perceived harmfulness of physical activities in patients with chronic low back pain: the Photograph Series of Daily Activities–short electronic version. J Pain. 2007;8:840–849.
- Zhan S, Ottenbacher KJ. Single subject research designs for disability research. Disabil Rehabil. 2001;23:1–8.
- Lundervold DA, Belwood MF. The best kept secret in counseling: single‐case (N = 1) experimental designs. J Couns Dev. 2000;78:92–102.
- Vet HCWd, Terwee CB, Mokkink LB, et al. Measurement in medicine: a practical guide. Cambridge (UK): Cambridge University Press; 2011.
- Lundberg MKE, Styf J, Carlsson SG. A psychometric evaluation of the Tampa Scale for Kinesiophobia - From a physiotherapeutic perspective. Physiother Theory Pract. 2004;20:121–133.
- Monticone M, Ambrosini E, Rocca B, et al. Responsiveness and minimal clinically important changes for the Tampa Scale of Kinesiophobia after lumbar fusion during cognitive behavioral rehabilitation. Eur J Phys Rehabil Med. 2017;53:351–358.
- Sullivan M, Karlsson J, Ware JE. Jr. The Swedish SF-36 health survey–I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41:1349–1358. Nov
- Sullivan MJ, Adams H, Rhodenizer T, et al. A psychosocial risk factor–targeted intervention for the prevention of chronic pain and disability following whiplash injury. Phys Ther. 2006;86:8–18.
- Scott W, Wideman TH, Sullivan MJ. Clinically meaningful scores on pain catastrophizing before and after multidisciplinary rehabilitation: a prospective study of individuals with subacute pain after whiplash injury. Clin J Pain. 2014;30:183–190.
- Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56.
- Sokka T. Assessment of pain in rheumatic diseases. Clin Exp Rheumatol. 2005;23:S77–S84.
- Asher AL, Kerezoudis P, Mummaneni PV, et al. Defining the minimum clinically important difference for grade I degenerative lumbar spondylolisthesis: insights from the Quality Outcomes Database. Neurosurg Focus. 2018;44:E2.
- Fairbank JCT, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976). 2000;25:2940–2953.
- Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20.
- Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J. 2010;19:1484–1494.
- Stratford P. Assessing disability and change on individual patients: a report of a patient specific measure. Physiother Can. 1995;47:258–263.
- Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical research council guidance. BMJ. 2015;350:h1258.
- Ziebland S, Featherstone K, Snowdon C, et al. Does it matter if clinicians recruiting for a trial don’t understand what the trial is really about? Qualitative study of surgeons’ experiences of participation in a pragmatic multi-centre RCT. Trials. 2007;8:4.
- Woby SR, Urmston M, Watson PJ. Self-efficacy mediates the relation between pain-related fear and outcome in chronic low back pain patients. Eur J Pain. 2007;11:711–718.