Abstract
Background
The main aim was to examine whether patients with persistent upper quadrant pain have higher end-point variability in goal directed pointing movements than pain-free controls when the pointing task is performed in total darkness and under full vision. An additional aim was to study associations between the magnitude of end-point variability and a clinical movement control test battery and self-rated functioning among patients.
Methods
Seventeen patients and 17 age- and gender-matched pain-free controls performed a pointing task that evaluated end-point variability of repetitive shoulder movements in horizontal adduction and abduction with full vision, and abduction with no visual information, completed a movement control test battery of neck and shoulder control tests and answered questionnaires.
Results
Patients had higher end point variability for horizontal abduction when performed with no visual information. For horizontal adduction the variability was higher, but only when it was controlled for movement time. No significant correlations were found between end-point variability and self-rated functioning, nor between end-point variability and neuromuscular control of the glenohumeral joint.
Conclusions
This study provides preliminary evidence that patients with persistent neck/shoulder pain can partly compensate proprioceptive deficits in goal-directed arm movement when visual feedback is present.
Introduction
Understanding patients’ functional impairments and activity limitations, and their potential origins is important for assessment and rehabilitation. Many patients with pain in the neck/shoulder region have problems performing daily activities involving hand and neck movements [Citation1,Citation2]. One of the many dynamic and interacting factors that may be associated with their compromised function and activity limitations could be altered movement coordination strategies [Citation3]. It is the central nervous system (CNS) that controls movements and joint stability (‘the joint remaining or promptly returning to proper alignment through an equalisation of forces’ [Citation4]). The CNS control requires well integrated information from both visual, vestibular and somatosensory systems, including proprioception. Proprioception involves conscious or unconscious awareness of joint position sense and is the product of afferent information from the mechanoreceptors in the muscle-tendon unit, joint, fascia and skin transmissioned to the CNS [Citation5]. It has earlier been shown that patients with acute and chronic musculoskeletal pain in the upper quadrant show impairments in proprioception [Citation6–12]. Notably, the information from the mechanoreceptors is integrated with visual and vestibular information and processed at many CNS-levels before activation of skeletal muscles [Citation13].
A daily task that involves proprioceptive inputs from the mechanoreceptors in the upper quadrant and integration of visual inputs is positioning of the hand [Citation14]. In a study examining a head-eye-hand coordination task performed with visual feedback (goal-directed arm movements), reduced precision was found in patients with chronic neck pain when compared to control subjects [Citation9]. Further, a strong association was found between end-point reaching acuity and self-rated symptoms and neck function. To our knowledge, no study has examined whether the same results can be expected if patients cannot rely on visual input.
Further, in a case control study [Citation15] including patients with recurrent episodes of non-traumatic mild neck pain, biomechanically less advantageous cervical extension, protraction-retraction and rotation movement patterns were found in patients when compared to pain-free study participants. Analysing movement impairments in the neck and shoulder regions can be a difficult task since the upper quadrant consists of complex muscle arrangements across joints. To evaluate neuromuscular control [Citation16–18] and muscles ability to evaluate control of movements in a specific direction [Citation15,Citation17–22] and to generate adequate and balanced force [Citation17,Citation20], clinical movement control tests have been introduced. It has been concluded that physiotherapists are able to reliably evaluate movement impairments [Citation20,Citation21].
To date, the relationships between goal-directed pointing performance, visual input, movement impairments evaluated using clinical movement control tests and subjective signs and symptoms have not been investigated. Since deficits in these aspects could play a role in the development of recurrent or chronic pain, and therefore are more relevant for rehabilitation than diagnostic labels naming pathological structures [Citation23], a deeper understanding of their nature is important. Analyses of associations between laboratory based tests, clinical movement control tests and self-rated functioning can reveal the clinical relevance of the sensorimotor impairments as well as provide leads to the mechanism behind the impairments.
The main aim of the present study was to examine whether patients with persistent upper quadrant pain have higher end-point variability in goal directed pointing movements than pain-free controls when the pointing task is performed in total darkness and under full vision. An additional aim was to study associations between the magnitude of end-point variability and a clinical movement control test battery and self-rated functioning among patients.
Methods
Design
This was a cross-sectional, laboratory, observer-blinded, controlled and comparative group study. It was approved by the Regional Ethical Review Board in Umeå, Sweden (08-083 M). Patients were informed that to decline participation in the study or to withdraw participation did not affect their rights to further rehabilitation. After the data collection was performed, patients were treated as in normal clinical practice. Written informed consent from each study participant was obtained.
Participants
Seventeen patients (five men and 12 women between 34 and 65 years, mean age 50 years) who sought medical care during a period of four weeks in a primary health care centre for their neuromusculoskeletal problems in the upper quadrant (cervicothoracic spine and upper limb) were recruited. Patients who fulfilled the inclusion criteria were consecutively asked by their physiotherapist if they wanted to participate in a study about signs and symptoms in patients with problems in their neck/shoulder region. Thereafter, information about the study, an informed-consent form, and questionnaires were sent to their home addresses, and they were scheduled for an appointment with the test leaders. All patients that were asked volunteered. Inclusion criteria was pain in the upper quadrant area ≥3 months in duration, and who were sub-classified by their physical therapist as having nociceptive mechanical pain as their dominating pain characteristic.
Exclusion criteria were red flags, visual analogue scale rating (VAS) >70 mm, major depressive disorder or substance abuse. Since one of the tests (the pointing task) included arm elevations above 110° and neck rotations more than 65°, patients with an active ROM below these thresholds were excluded. A goniometer was used to asses ROM.
Each patient was matched with a pain-free study participant (controls) of the same age (±1 year) and sex. Inclusion criteria for the control group included no health complaints or pain in the upper quadrant during the last 12 months, no known history of neck or shoulder injury that required medical treatment, full ROM of the neck and being able to move their shoulders to the target positions without discomfort or limitations. The pain-free study participants were recruited through advertising at the University of Umeå.
Data collection
Two testers, blinded to whether the study participants were patients or pain-free controls, performed and rated the tests together.
Goal-directed pointing task
This task, when pointing with the right hand to a target diagonally to the left (hereafter called Adduction with vision), has been described in detail earlier [Citation9]. In the present study, we added two new conditions: pointing to the right under full vision (Abduction with vision) and pointing to the right in total darkness (Abduction in darkness). The reason for also including abduction was that abduction involves higher biomechanical demands (the arm and hand moves away from the centre of body mass with an increased lever arm affecting the muscles and joints in the trunk, shoulder and cervical area). It also involves an increased tension and mechanical load on the brachial plexus. We hypothesised that removal of visual feedback would increase end point variability to a greater extent in the patient group compared to the control group.
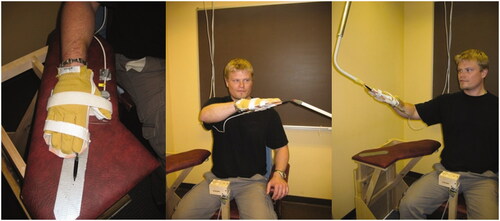
The test procedure and data collection were fully automatised and instructions were pre-recorded and presented through speakers. The participant was seated in a rigid chair with their pelvis strapped to the back of the chair to isolate movements to the shoulder and arm. An arm support was placed at waist height on the right side of the chair. A marker was placed on the arm rest as a guide for how to place the hand in the starting position (). A pointer fixated to a rectangular plastic plate was firmly attached to the hand. The pointer was fixed in line with the third digit, extending 20 cm from the fingertip. The two targets had both a soft foam-rubber stick, one cm in diameter. The target to the left was placed in front of the study participant at the distance corresponding to the location of the wrist of the participant’s left extended arm at eye level height and 20 cm to the left of the participant’s left acromion. The target to the right was placed at the end of the participant’s extended third digit, with the right arm extended at eye level and horizontally abducted 35°.
The study participant was given the following instructions; ‘The task is to place the pointer as close as possible to the target. You should do this as fast and accurately as possible. When you have reached the target, keep the pointer still for a few seconds and do not correct the position’. Three to five practice trials were allowed. A command ‘Go’ indicated to start the pointing movement and ‘Go to the starting position’ the return. This procedure was repeated 15 times. In a random order, the participants started with the full vision condition or the darkness condition.
Kinematic data was recorded with an electromagnetic tracking system (FASTRAK, Polhemus Inc., USA) at a sampling rate of 30 Hz. The global coordinate system had an orientation such that its coordinate axes X, Y and Z, respectively, corresponded to the horizontal, depth and vertical directions in relation to the body. Pointer coordinates were calculated from data collected by a sensor attached to the hand plate. Movement initiation and termination were assessed from the velocity profiles of the pointer tip.
Outcome measures were end-point variability and movement time. End-point variability was defined as the mean ellipse volume (cm3) based on the Variable Error (VE) of the pointer tip position at the time of movement termination, calculated separately along each coordinate axis (X, Y and Z) [Citation24]. VE was calculated as the population standard deviation of the algebraic errors (distance between pointer and target) for the 15 trials [Citation9]. The movement time was calculated for each trial and movement times averaged over all 15 trials for each testing condition were used in the analyses.
Movement control test battery
All tests but one (seated scapula/glenohumeral medial and lateral rotation test, part B: 20 fast rotations, see below) have been described in detail earlier [Citation20]. The tests were performed in one sequence, as is customary in clinical practice. The verbal instructions were standardised [Citation20]. The participants were given a minimum of cues so that they performed their preferred pattern of movement. All tests but the seated scapula/glenohumeral medial and lateral rotation test part B, were scored as ‘positive’ (movement impairment) or ‘negative’ (performed the test correctly according to defined criteria). The tests were observed for 4–6 repetitions before it was scored positive or negative.
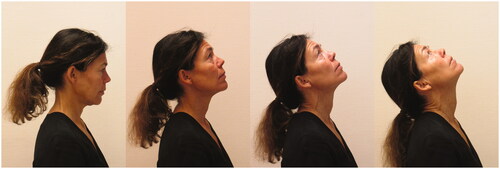
The seated head turn test assesses the ability to move the cervical spine into rotation of up to 80° without cervical side-bend [Citation25] (). The test was evaluated as negative if there was a symmetrical range of rotation to 60–70° with eyes horizontal (no lateral flexion) and no chin poke (indicating a concurrent extension of the cervical spine during the rotation). It was evaluated as positive if there was an uncontrolled movement seen as (1) a chin poke [Citation18] and/or (2) lateral flexion and/or (3) restricted range of motion (ROM <75° from the midline with eyes horizontal) and/or (4) jerkiness (the movement did not look smooth and easy). Inter-rater reliability: k = 0.78 and k = 0.89, percentage agreement 89 and 94% for the right and left side, respectively [Citation20]. The following instructions were used: Make a straight back. Turn your head and neck slowly to the right and back to the starting position. Try to make the rotation around an axis that runs longitudinally through your head, neck and spine. The test was rated separately for the right and left side, respectively. In the analyses, only the right side was included.
Figure 2. Photograph of the seated head turn test. Illustrating the starting position and rotation to the left.

The seated neck extension test assesses the quality of natural neck extension in sitting (). The test was evaluated as negative if the lower and upper cervical spine was contributing to extension equally to 15–20°. It was evaluated as positive if (1) there was an excessive upper cervical extension seen as a chin poke and head back over the base of support (cervico-thoracic extension is performed late or not at all during the movement) and/or (2) during the return from extension the upper cervical stayed in extension and/or (3) there was an excessive mid-cervical anterior translation during the active extension (seen as a skin crease transversely across the posterior neck at the level of the anterior translation) and/or (4) restricted range of motion (ROM < 15°) and/or (5) there was jerkiness. Inter-rater reliability: k = 0.66, percentage agreement 86% [Citation20]. The following instructions were used: Make a straight back. Slowly look up to the ceiling and back to the starting position.
Figure 3. Photograph of the seated neck extension test. Illustrating start position (A) and the individual neck extension movement pattern (B–D).
The seated scapula/glenohumeral rotation test, part A assesses the quality and symmetry of glenohumeral rotation. The test was modified to be performed in sitting instead of supine [Citation16,Citation18]. With a 90° abduction in the glenohumeral joint, humerus in the scapular plane and 90° flexion in the elbow the participants were asked to actively medially and laterally rotate to their maximum and then return to the starting position (same set-up for part A and part B, ). This test had two outcome measures. Positive/negative and degrees of medial rotation. The test was evaluated as negative if the participant could medially and laterally rotate the glenohumeral joint while preventing compensating movements in the glenohumeral joint (humeral head anterior translation) and of the scapula (forward tilt, elevation, downward rotation). It was evaluated as positive if (1) the shoulder rolled forward (forward tilt/elevation of the scapula) before the participant achieved 50–60° medial rotation or 90° lateral rotation of the glenohumeral joint and/or the humeral head glided excessively in any noticeable direction during medial rotation (UCMs) and/or (2) restricted range of motion (ROM medial rotation 50–60°, lateral rotation 90°) and/or (3) there was jerkiness. Inter-rater reliability: k = 0.37, percentage agreement 92% [Citation20]. For instructions, the test leader first showed the starting position and the movement. Then the following instructions were used: Rotate your shoulder so that the hand goes down to the maximum. Then rotate your shoulder so that the hand moves towards the ceiling to the maximum. And then return to the starting position. Try to make the rotation around an axis that runs longitudinally through your upper arm.
Figure 4. Photograph of the seated scapula/glenohumeral rotation test B. Illustrating the starting position and the movements during 20 fast rotations.
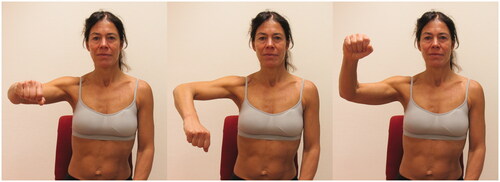
The seated scapula/glenohumeral medial and lateral rotation test, part B: 20 fast rotations assesses the ability to perform fast rotations in the glenohumeral joint (the single outcome measure is time in seconds, ). This test is used in clinical settings in Sweden for evaluation of shoulder function (ability to control movements such as anterior or superior glide in the glenohumeral joint during sports activity). Information about test-retest reliability and normative values is incomplete. The participant was asked to actively medially and laterally rotate their shoulder 20 times as fast as possible (s) within their full individual ROM and without the testers controlling how they performed the rotations. In cases when the participant was not able to complete all 20 repetitions, the time used for the number of completed rotations was used to calculate the time for one trial, from where an estimation of the time for 20 repetitions was done (time/rep × 20). The following instructions were used: Now, rotate your shoulder 20 times as fast as you can within the range of motion you have.
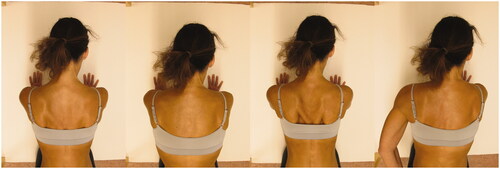
The scapular abduction–adduction in four-point kneeling assesses the ability of the Serratus Anterior muscle to actively shorten through the available range of scapula abduction with upward rotation and thereby stabilise the scapula to the thorax wall during repeated scapular abduction–adduction (×10) followed by a 5 s isometric contraction in the end position (). The test was evaluated as negative if the participant could hold the position for 5 s with the medial border of the scapula (at the level of the spine) at least 10 cm from the spine, in contact with the thorax without substitution by other muscles or fatigue. It was evaluated positive if there was visible substitution by other muscles (scapula wings, scapular tilt, scapular elevation, scapular downward rotation, scapular depression or spinal flexion, or trunk rotation) or the participant could not hold the position for 5 s in the optimal position (without obvious scapula winging/tilting/elevation/downward rotation/scapular depression). Inter-rater reliability: k = 0.76, percentage agreement 86% [Citation20]. First, the test leader showed the starting position and the movement. Then the following instructions were used: Please put the load of your body on the arms, and then smoothly and in a self-selected pace, repeatedly (×10) elevate your chest by making the shoulder blades wide and lower your chest by letting the shoulder blades return to starting position. After doing so, please stop and hold this elevated position for 5 s. Please do not bend your arms or move backward during the movements.
Pain measurement and questionnaires
Visual Analog Scale (VAS) [Citation26] was used to assess the patients’ pain in the neck/shoulder region ‘during the last seven days’. The VAS ranges from 0–100 mm, with 0 mm representing ‘no pain at all’ and 100 mm the ‘worst imaginable pain’.
The disability of the arm, shoulder and hand questionnaire (DASH) [Citation2] was used to measure disability and symptoms in daily life. It consists of 30 items where each item is scored on a 1- to 5-point scale, ranging from ‘no difficulty’ or ‘no symptom’ to ‘unable to perform activity’ or ‘very severe symptom’. A higher score indicates more disability. Total score 150.
The Neck Disability Index (NDI) [Citation1] was used to measure the severity of disability in ten different activities; personal care, lifting, reading, work, driving, sleeping, recreational activities, pain intensity, concentration and headache. A higher score indicates more disability. There are six response alternatives for each item, ranging from no disability (0) to total disability (5). A higher score indicates more pain and disability. Total score 50.
The Tampa Scale of Kinesiophobia (TSK) [Citation27] was used to assess the fear of re-injury due to movement. The TSK is a 17-item questionnaire where each item is rated on a 4-grade Likert scale with scoring alternatives ranging from ‘strongly agree’ to ‘strongly disagree’. A higher score indicates more kinesiophobia. Total score 68.
Statistical analyses
The Wilcoxon signed rank test for related samples was used to compare questionnaire data, degrees of medial rotation during the seated scapula/glenohumeral rotation test part A and performance time for the 20 fast rotations during part B, end-point variability and movement time for the goal directed pointing tasks between the age- and sex-matched groups. McNemar Test was used to compare performance of the single movement control tests (positive/negative). Odds Ratio (OR) for showing a movement impairment if you are a patient has also been calculated using the Chi-square test.
To adjust for speed-accuracy trade-off effects, which would influence the precision at the end point in movement tasks like ours [Citation28], additional analyses were performed for end-point variability to control for the effect of movement time. In a linear regression model of end-point variability (Y) and movement time (X) the residuals of the fitted trend line were calculated representing variability controlled for movement time, which was analysed for differences between the groups.
Spearman’s correlation was used to analyse relationships between self-rated functioning, end-point variability and ‘Seated scapula/glenohumeral medial and lateral rotation’ among patients.
A statistician performed a sample size calculation to verify the ability to detect group differences for the proportions of positive movement control tests with the McNemar test [Citation29]. With a prevalence of 65% in one group and 25% in the other, a power of 80% and α = 0.05, we needed 17 participants in each group.
Results
Characteristics of patients and pain-free controls are presented in .
Table 1. The results from the pain measurement and questionnaires among the patients with upper quadrant pain (patients, n = 17) and the pain-free controls (controls, n = 17).
shows the results of goal-directed pointing. Patients showed similar end-point variability in the light on conditions, but not when abduction was performed in the darkness. In darkness, the end-point variability was greater in patients than in controls (p = 0.015). Notably, when controlled for movement time, end-point variability showed significant differences between groups for both Adduction with vision (p = 0.022) and Abduction in darkness (p = 0.044), while Abduction with vision did not reach significance (p = 0.084) (not shown in table).
Table 2. Results of goal-directed pointing; median (min–max) of end-point variability and movement time for the patients with persistent upper quadrant pain (patients, n = 17) and the pain-free controls (controls, n = 17).
shows results from the movement control test battery. According to the predefined criteria for neck movements, more patients than controls showed restricted ROM. During active neck extension, more patients also showed an excessive mid-cervical anterior translation and their movements did not look smooth and easy. Two patients could not perform 20 fast rotations in the glenohumeral joint.
Table 3. Comparisons of the outcomes of the tests included in the movement control test battery between the patients with persistent upper quadrant pain (patients, n = 17) and the pain-free individuals (controls, n = 17).
The associations between end-point variability and degrees of medial rotation, performance time for 20 fast shoulder rotations and self-rated functioning in patients are shown in . No significant correlations were found between end-point variability and self-rated functioning, nor between end-point variability and the measures of neuromuscular control of the glenohumeral joint.
Table 4. Associations (Spearman’s correlations, rs) between end-point variability, the seated scapula/glenohumeral medial and lateral rotation test and self-rated functioning in patients with persistent upper quadrant pain (n = 17).
Discussion
This study demonstrates that people with upper quadrant pain can have higher end-point variability in goal-directed reaching than healthy controls. The acuity reduction was most prominent when they pointed in the non-vision condition. The reason for the higher end-point variability among patients could be expected as upper quadrant pain may lead to impaired proprioception [Citation7,Citation9,Citation30]. When participants’ variability was controlled for the effect of movement time, significant differences between groups were found for both vision (when pointing in adduction) and the non-vision condition. Notably, movement time did not confound movement accuracy in darkness. Possibly because proprioceptive deficits in patients with upper quadrant pain [Citation7] surface when visual feedback cannot be used to control the movement.
When analysing the associations between end-point variability and self-rated functioning in patients, we found no significant correlations. As discussed previously, it is possible that vision may compensate for potentially reduced somatosensory feedback, that is, joint positional sense, and thus normalise pointing variability. Neither did we find any significant correlations between end-point variability and the range of glenohumeral medial rotation or the ability to perform fast rotational movements in the glenohumeral joint. Instead we found significant correlations of moderate effect size between arm and hand (DASH) and neck (NDI) disability and aforementioned shoulder tests. The ability to perform these tests were also clearly reduced in patients when compared to healthy controls. In our study, the rotational range was ∼20° reduced. As described earlier, a decreased range of glenohumeral medial rotation can be an indicator of impaired scapular control [Citation19] and decreased muscle length or increased stiffness in the posterior deltoid, infraspinatus, teres minor, and/or the posterior capsule [Citation31]. It has been explained that if these muscles are too short or stiff, they might contribute to humeral anterior glide during overhead motions and horizontal adduction [Citation31,Citation32]. This alteration in the precision of patients’ shoulder movements might in turn have contributed to pain and inability to perform fast rotational movements.
The fact that patients showed slightly more movement impairments during the tests of movement control in the cervical spine and gleno-humeral joint when compared to the pain-free study participants is in accordance with an earlier study investigating prevalence of movement impairments in patients with neck pain [Citation15] and with low back pain [Citation33]. This strengthens the importance of including tests of movement patterns in the examination as suggested earlier [Citation16–19,Citation34,Citation35]. It has to be stressed though that, as shown in this study (), a test can be scored positive due to various reasons and it is important to further examine what specific movement impairment a patient shows. A movement impairment could be due to altered muscle recruitment patterns [Citation30,Citation36,Citation37] (e.g. when there is an excessive mid cervical anterior translation during neck extension [Citation18,Citation20]) and/or relatively more stiffness in one joint/segment compared to another (e.g. a chin poke during neck rotation, when there is relatively more stiffness in the lower cervical spine than in the upper cervical spine), muscle shortness or decreased strength [Citation16,Citation17,Citation19]. For example, in the seated neck extension test, where a high percentage of the participants did not correctly perform the test, patients showed more movement impairments including lower ROM, inability to control mid cervical anterior translation and inability to extend the neck smoothly. These latter movement impairments likely reflect an altered movement strategy for the task, such as a reduced deep cervical flexor activity. A reduced activity of the deep cervical flexor muscles in patients with neck pain has been shown earlier [Citation38] and this ability is often evaluated using the craniocervical flexion test [Citation39]. Another matter that has to be stressed is that the primary focus of these movement control tests is their association with symptoms [Citation16,Citation17,Citation19,Citation23]. When using the tests in clinical practice, first a patient’s preferred alignment or movement strategy during the movement test is observed. If an impairment of alignment or movement is observed and the patient expresses a symptom response, the test is repeated with an appropriate correction/modification and the patient’s response is re-evaluated for symptoms and/or quality of motion [Citation16,Citation17,Citation19]. Upon completion of several tests, the findings are reviewed to determine if there is a consistent pattern of symptom responses (increased or decreased) associated with a specific movement direction. Consistency of responses leads to the focus of physiotherapy treatment [Citation16,Citation17,Citation19].
Some of the movement impairments investigated in this study were also evident among the pain-free study participants. These findings suggest that movement system impairments are not single, isolated events but are common and probably affected by lifestyle. According to the kinesiopathological model, they could, potentially, hold the risk for future development of clinical symptoms not yet manifested among the pain-free study participants [Citation16,Citation17,Citation23]. However, a direct relationship between positive test findings and future health deficits can only be determined with prospective studies. Accordingly, it has been suggested that disability and human movement should be interpreted in the framework of complex adaptive (or dynamical) systems, and that movement behaviour, health and pain development thus depend on intricate interactions of multiple factors of the individual and the environment [Citation40].
We have a few methodological considerations. Firstly, we included patients who actively sought care at an outpatient physical therapy clinic for persistent pain in the neck/shoulder area. This strengthens the generalisability regarding patients seeking care, but might not be generalisable to other populations of people with upper quarter pain. Secondly, the pain-free study participants who were selected as controls were matched regarding sex and age, but not to any lifestyle factors such as physical activity level, or psychosocial factors such as fear avoidance or kinesiophobia, which might influence the results. Thirdly, the seated neck extension test evaluated both eccentric (during neck extension) and concentric (during the return) control of the deep neck flexors, which is different from other studies [Citation22]. Fourthly, the participants were asked to place the pointer as fast and accurately as possible. Some participants might have prioritised to do it as accurate as possible whereas others as fast as possible. Fifthly, many findings were significant, but the results of this study must be interpreted in light of the sample size of 34 participants and possible risks of type I errors.
Conclusion
This study provides preliminary evidence that patients with persistent neck/shoulder pain can partly compensate proprioceptive deficits in goal-directed arm movement when visual feedback is present. There were significant differences between patients and pain-free study participants in their ability to accurately reach the target, neck movement patterns and degrees of shoulder medial rotation. Considering the size of the differences and size of the variability found in the controls, these findings have to be investigated further before conclusions can be drawn.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–415.
- Hudak PL, Amadio PC, Bombardier C, et al. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand). Am J Ind Med. 1996;29(6):602–608.
- Mottram S, Blandford L. Assessment of movement coordination strategies to inform health of movement and guide retraining interventions. Musculoskelet Sci Pract. 2020;45:102100.
- Riemann BL, Lephart SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002;37(1):71–79.
- Roijezon U, Clark NC, Treleaven J. Proprioception in musculoskeletal rehabilitation. Part 1: basic science and principles of assessment and clinical interventions. Man Ther. 2015;20(3):368–377.
- Treleaven J, Jull G, Sterling M. Dizziness and unsteadiness following whiplash injury: characteristic features and relationship with cervical joint position error. J Rehabil Med. 2003;35(1):36–43.
- Sandlund J, Djupsjobacka M, Ryhed B, et al. Predictive and discriminative value of shoulder proprioception tests for patients with whiplash-associated disorders. J Rehabil Med. 2006;38(1):44–49.
- Juul-Kristensen B, Lund H, Hansen K, et al. Poorer elbow proprioception in patients with lateral epicondylitis than in healthy controls: a cross-sectional study. J Shoulder Elbow Surg. 2008;17(1):72S–81S.
- Sandlund J, Roijezon U, Bjorklund M, et al. Acuity of goal-directed arm movements to visible targets in chronic neck pain. J Rehabil Med. 2008;40(5):366–374.
- Sjolander P, Michaelson P, Jaric S, et al. Sensorimotor disturbances in chronic neck pain-range of motion, peak velocity, smoothness of movement, and repositioning acuity. Man Ther. 2008;13(2):122–131.
- de Vries J, Ischebeck BK, Voogt LP, et al. Joint position sense error in people with neck pain: a systematic review. Man Ther. 2015;20(6):736–744.
- Reddy RS, Tedla JS, Dixit S, et al. Cervical proprioception and its relationship with neck pain intensity in subjects with cervical spondylosis. BMC Musculoskelet Disord. 2019;20(1):447.
- Shumway-Cook A, Woollacott MH. Motor control: translating research into clinical practice. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012.
- van Beers RJ, Wolpert DM, Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Curr Biol. 2002;12(10):834–837.
- Elsig S, Luomajoki H, Sattelmayer M, et al. Sensorimotor tests, such as movement control and laterality judgment accuracy, in persons with recurrent neck pain and controls. A case-control study. Man Ther. 2014;19(6):555–561.
- Sahrmann SA. Diagnosis and treatment of movement impairment syndromes. St. Louis (MO): Mosby; 2002.
- Sahrmann S. Movement system impairment syndromes of the extremities, cervical and thoracic spines. St. Louis (MO): Elsevier Mosby; 2011.
- Comerford M, Mottram S. Kinetic control: the management of uncontrolled movement. St. Louis (MO): Elsevier; 2012.
- Caldwell C, Sahrmann S, Van Dillen L. Use of a movement system impairment diagnosis for physical therapy in the management of a patient with shoulder pain. J Orthop Sports Phys Ther. 2007;37(9):551–563.
- Aasa B, Lundström L, Papacosta D, et al. Do we see the same movement impairments? The inter-rater reliability of movement tests for experienced and novice physiotherapists. Euro J Physiother. 2014;16(3):173–182.
- Patroncini M, Hannig S, Meichtry A, et al. Reliability of movement control tests on the cervical spine. BMC Musculoskelet Disord. 2014;15:402.
- Segarra V, Duenas L, Torres R, et al. Inter-and intra-tester reliability of a battery of cervical movement control dysfunction tests. Man Ther. 2015;20(4):570–579.
- Sahrmann S, Azevedo DC, Dillen LV. Diagnosis and treatment of movement system impairment syndromes. Braz J Phys Ther. 2017;21(6):391–399.
- Adamovich SV, Archambault PS, Ghafouri M, et al. Hand trajectory invariance in reaching movements involving the trunk. Exp Brain Res. 2001;138(3):288–303.
- Kapandji I. The physiologoy of the joints: the trunk and the vertebral column. Volume 3. London (UK): Churchill Livingstone; 1974.
- Huskisson EC. Measurement of pain. Lancet. 1974;304(7889):1127–1131.
- Kori S, Miller R, Todd D. Kinesiophobia: a new view of chronic pain behaviour. Pain Management. 1990;3:35–43.
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47(6):381–391.
- Connor R. Sample size for testing differences in proportions for the paired-sample design. Biometrics. 1987;209(1):207–211.
- Falla D, Farina D. Neural and muscular factors associated with motor impairment in neck pain. Curr Rheumatol Rep. 2007;9(6):497–502.
- Ludewig PM, Cook TM. Translations of the humerus in persons with shoulder impingement symptoms. J Orthop Sports Phys Ther. 2002;32(6):248–259.
- Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136–151.
- Luomajoki H, Kool J, de Bruin ED, et al. Movement control tests of the low back; evaluation of the difference between patients with low back pain and healthy controls. BMC Musculoskelet Disord. 2008;9(1):170.
- Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276–291.
- Ludewig PM, Lawrence RL, Braman JP. What’s in a name? Using movement system diagnoses versus pathoanatomic diagnoses. J Orthop Sports Phys Ther. 2013;43(5):280–283.
- Falla D, Bilenkij G, Jull G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine. 2004;29(13):1436–1440.
- Falla D, Farina D. Neuromuscular adaptation in experimental and clinical neck pain. J Electromyogr Kinesiol. 2008;18(2):255–261.
- Falla DL, Jull GA, Hodges PW. Patients with neck pain demonstrate reduced electromyographic activity of the deep cervical flexor muscles during performance of the craniocervical flexion test. Spine. 2004;29(19):2108–2114.
- Jull G, Falla D. Does increased superficial neck flexor activity in the craniocervical flexion test reflect reduced deep flexor activity in people with neck pain? Man Ther. 2016;25:43–47.
- Brown CA. The role of paradoxical beliefs in chronic pain: a complex adaptive systems perspective. Scand J Caring Sci. 2007;21(2):207–213.