Abstract
Purpose
To describe hand function, and investigate adherence to a rehabilitation protocol and factors associated with upper extremity disability in non-surgically treated proximal phalangeal fractures.
Material and methods
In a prospective cohort study, 86 patients (58 women, mean age of 49 years) were assessed at cast removal and 6 weeks follow-up. Adherence was registered in a diary. Factors potentially associated with disability were assessed in a logistic regression model.
Results
At 6 weeks, the mean total active range of motion in the injured finger was 84% of the uninjured finger and median pain intensity levels were low (VAS <20). Mean grip strength was 66% of the uninjured hand and 28% perceived high upper extremity disability (QuickDASH score ≥30). Most patients adhered to exercise and night splint regime. The strongest associated factors with high upper extremity disability were more days in cast (Odds ratio 1.429, 95% CI 1.110–1.840) and fear of movement (Odds ratio 1.119, 95% CI 0.990–1.256) in the final regression model (Nagelkerke R Square 0.46).
Conclusion
Most patients regain early satisfactory hand function, but a quarter still perceives high upper extremity disability. Longer immobilisation time in particular and fear of movement are important factors that may negatively affect the early outcome.
Introduction
Hand fractures are among the most common fractures in the general population, and, of these, 50 percent are phalangeal fractures, particularly involving the proximal phalanx (P1) [Citation1,Citation2]. Most P1 fractures can be managed conservatively, i.e. non-surgically treated, even in some unstable cases [Citation3] and the fracture will be healed in about 6 weeks [Citation4]. After any required reposition, the P1 fracture is normally immobilised in a cast for 2–3 weeks and the hand is placed in an intrinsic-plus position. The extensor apparatus is thereby tightened, which stabilises the fracture [Citation5,Citation6].
The consequences after a P1 fracture can be classified according to the International Classification of Functioning, Disability, and Health (ICF) [Citation7]. The term functioning refers to all body functions and structures, activities, and participation, while disability is similarly a term for impairments, activity limitations, and participation restrictions. The goal of rehabilitation following a P1 fracture is to re-establish hand function with painless mobility, strength, and the ability to perform upper extremity daily activities. Early interventions during the first 6 weeks include oedema control, splint regime, and range of motion exercises [Citation8,Citation9]. Increased load during activities and strengthening exercises are included once adequate fracture healing has occurred to enable a return to usual activities and work [Citation8,Citation9].
Previous studies on non-surgically treated P1 fractures have reported a satisfactory outcome of a range of motion and pain in follow-up assessments 1–2 years after injury [Citation10–12]. A study by Franz et al. [Citation13] compared immobilisation of P1 fractures in two different types of casts, one including the wrist and the other leaving the wrist free. Both groups wore the cast for 4 weeks and both casts blocked the metacarpophalangeal (MCP) joints in flexion, but the interphalangeal (IP) joints were free to be actively moved. At 4 weeks of follow-up better wrist motion for the patients with the hand-based cast was found, and at 12 weeks both groups showed good return of joint motion, suggesting that effective treatment can be achieved without immobilising the wrist. In a recent study by Byrne et al. [Citation14], patients with P1 fractures were immediately placed in a hand-based thermoplastic splint only immobilising the MCP joints. The participants followed a standardised rehabilitation protocol and regained good to excellent active joint motion and low levels of pain at discharge from rehabilitation. However, the time point for follow-up varied from 3 to 15 weeks, mean 7 weeks, after injury. These previous studies only reported outcomes of range of motion and pain, but no investigation of activity limitations or adherence to the rehabilitation protocol [Citation14]. As hand fractures are common and most patients are of working age, it involves high costs not only for the health care system but also to society related to sick leave during healing and rehabilitation [Citation15,Citation16]. More knowledge is therefore needed on early rehabilitation with a broader perspective of outcome both on levels of function and activity.
Several factors can affect recovery and rehabilitation after a hand fracture, such as the structural severity of the injury, the fracture type, the immobilisation period [Citation5,Citation6,Citation17], and age and sex [Citation1,Citation2]. Pain as well as behavioural factors, such as fear of movement, might also impact the rehabilitation process and contribute to disability after hand injuries [Citation18–21]. There is a lack of studies that have specifically investigated several factors’ association with outcomes after non-surgically treated P1 fractures. Improved knowledge about factors associated with the disability of the upper extremity after a non-surgically treated P1 fracture will enhance the ability to provide efficient rehabilitation and identify individuals early on in need of targeted interventions to avoid prolonged disability and rehabilitation periods.
The aim of the study was to describe hand function 6 weeks following non-surgically treated P1 fractures, and investigate adherence to a rehabilitation protocol and factors associated with upper extremity disability.
Methods
Participants
In this prospective longitudinal cohort study, patients were recruited from 2015 to 2018 at the Department of Hand Surgery, Skane University Hospital, Malmö, Sweden, and consecutively asked to participate. We included patients, with age of 18 years or older, who presented with 1 or 2 proximal phalangeal fracture(s) in the hand, either in the base, shaft, or distal metaphysis, where the function of the flexor and extensor tendons, as well as the digital nerves and vessels, were intact. Exclusion criteria were: other diseases or injuries that may affect upper extremity functioning, inability to understand and follow test instructions due to communication, mental or cognitive impairments. No patients with surgically treated fractures were included.
The fractures were initially treated in the emergency room at the hospital and judged as stable, or initially displaced and after reduction judged as stable. No fracture(s) with an articular step at the joints, being open or pathological, or having a rotation that could not be corrected through closed reduction were included. The plain radiographs were later judged and graded by a hand surgeon (LBD) as a non-displaced transverse fracture; non-displaced oblique fracture; non-displaced comminuted fracture; displaced transverse fracture, displaced oblique fracture; displaced comminuted fracture; or an intraarticular fracture without any step(s) at the articular surface.
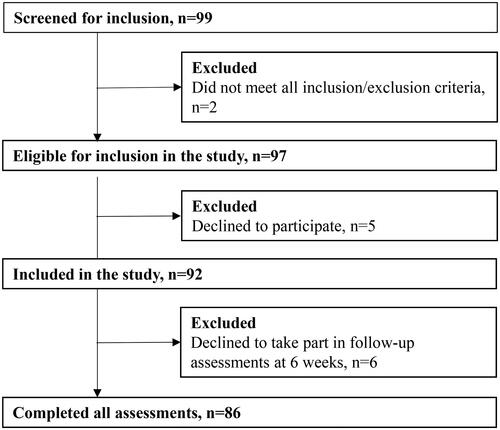
Ninety-seven patients were eligible for the study and five declined to take part. Ninety-two participants were therefore included in the study. Another six patients declined to participate in the follow-up at 6 weeks, leaving a total of 86 that completed all assessments in the study (flow chart in ).
Figure 1. Study flow-chart.
The study was approved by the Regional Ethical Board in Lund (Dno: 2014/827) and written informed consent was obtained from each participant before participation in the study.
Method of non-surgical treatment
According to local routines, patients with hand injuries are admitted to the emergency department at the hospital. If a fracture is confirmed by X-ray, initial reposition of a displaced fracture, if needed, is performed and position confirmed by another X-ray. The hand is immobilised in a forearm-based cast with the MCP joints flexed as close to 90° as possible and the proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints in an extended position. The patients are then referred to the Department of Hand Surgery, usually within 1 week, for further judgement of treatment, being either a surgical option or a non-surgical treatment.
If conservative non-surgical treatment is decided upon, the patients are referred to the rehabilitation unit at the Hand Surgery Department for cast removal and the start of rehabilitation at an appropriate time according to the treating hand surgeon’s decision, usually 2–3 weeks after the injury according to present clinical routines and agreements. If the patients suffer from persistent pain, due to suspected instability or other fracture-related problems, contact with the responsible hand surgeon is made for an additional assessment and X-ray.
Rehabilitation protocol
On the day of cast removal, rehabilitation starts according to a standardised program with an individual approach. The patients meet with an experienced physiotherapist (in the current study, other than the assessor), teaching them a specific home exercise program starting with an oedema-reducing exercise involving the shoulders. The program continues with controlled and careful passive and active range of motion exercises for the fingers and active range of motion exercises for the wrist within the pain limit. The patients are instructed to hold for a few seconds at the end range of motion and repeat five times. The whole exercise program is to be completed 7–8 times per day (every second hour). For detailed information, see Supplementary Material.
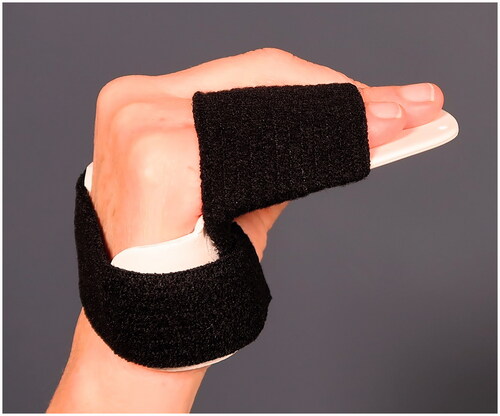
An occupational therapist provides the patients with a hand-based custom-made thermoplastic splint () facilitating effective MCP-joint flexion and PIP-joint extension and for protection of the fracture. The splint should be worn at night and in unprotected environments until follow-up at 6 weeks. Depending on the patients’ needs, the number of visits to the rehab unit until follow-up may vary.
Figure 2. Hand-based thermoplastic splint to be worn at night and in unprotected environments from cast removal until follow-up at 6 weeks.
From the day of cast removal, the patients may use their hand in light pain-free activities and are advised not to engage in sports, or heavy loading until at least 6 weeks has passed. A therapeutic approach to achieve good adherence is sought to ensure that each patient comprehends and agrees to follow the described exercises, the splint use, and load restrictions. This includes measures, such as verbal information about the purpose of the splint use, and verbal and written information, including a brochure with pictures of each exercise. Patients are also asked to carry out the exercises independently to ensure that they understand the instructions and are encouraged to ask any questions concerning their rehabilitation.
Outcomes
Range of motion
Active range of joint motion was measured in the injured finger/fingers with a finger goniometer (Saehan Corporation, Pakistan) according to standardised guidelines [Citation22,Citation23]. IP-joint extension deficits were measured and total active motion (TAM) was calculated as the total active flexion of the MCP, PIP, and DIP joints minus the total extension deficit of the same joints in degrees [Citation24]. The percentage of the uninjured finger on the other hand was calculated as TAM%. A TAM% >75% was considered as good [Citation24]. When 2 fingers were injured, the TAM of the worst injured finger was used.
Pain
The Visual Analogue Scale (VAS) was used to estimate the patients’ pain intensity, at rest, during activities, and after activities. The pain was assessed between 0 (no pain) and 100 (worst pain imaginable) [Citation25].
Grip strength
Grip strength was assessed with the use of a hydraulic dynamometer JAMAR (TEC, Clifton, New Jersey, US) according to standardised instructions [Citation23,Citation26]. The average of three efforts was calculated (kg) and also the percentage of the grip strength of the uninjured hand (grip strength%).
Disability of the upper extremity
Disability of the upper extremity was assessed by the QuickDASH questionnaire, a shorter version of DASH (Disability of Arm, Shoulder, and Hand) [Citation27]. QuickDASH is proven to be valid, reliable, and responsive in musculoskeletal conditions of the upper extremity [Citation28]. The QuickDASH questionnaire includes 11 items of which seven items concern the ability to perform different activities, one concerns participation, and three items concerns symptoms (such as sleeping difficulties and tingling sensations). Each item is scored from 1 = No difficulty to 5 = Unable. The total score ranges are indexed from 0 and 100, where a higher score reflects greater disability. A score >10 is reported to indicate some upper extremity disability, a score of 20 can be considered a moderate disability, and 30 high upper extremity disability [Citation29–31].
Fear of movement
Fear of movement or kinesiophobia (avoiding movement because of fear of (re)injury or pain) [Citation32] was evaluated by the Tampa Scale (Swedish version) [Citation33,Citation34]. The scale, originally developed for patients with chronic low back pain but increasingly used for other conditions, is found to be valid and reliable [Citation34]. The TAMPA scale consists of 17 statements concerning fear of movement and pain that are rated on a 4 point scale. The lowest score is 17 and the highest 68, which also is the highest degree of fear of movement. A score of ≥37 can be used as a cut-off, signifying a high fear of movement [Citation35].
Self-efficacy
Self-efficacy was assessed by the General Self-efficacy Scale, Swedish version (S-GSE) [Citation36–38]. The GSE scale, which consists of 10 items concerning the self-perceived ability to respond to novel or difficult situations and to deal with any associated obstacles or setbacks, has been found to be valid and reliable [Citation36,Citation38]. The items are rated on a 4 point scale (‘not at all true’ to ‘exactly true’). The total score ranges from 10 to 40 points. The higher score the higher self-efficacy and ≥30 has been suggested to indicate high self-efficacy [Citation39].
Procedures
At cast removal, the participants rated their pain at rest (VAS) and fear of movement (TAMPA scale) and were instructed to report the number of exercise sessions completed and night splint use in a diary. At 6 weeks follow-up, personal data were collected together with the exercise and splint diary. Patients rated their pain at rest and during and after activities (VAS), fear of movement (TAMPA scale), self-efficacy (GSE scale), and upper extremity disability (QuickDASH). Active joint motion and grip strength were measured. The same order of assessment, described above, was used for all participants. Information on the P1 fracture was extracted from the medical records and the fracture type (non-displaced or displaced) was confirmed by an experienced hand surgeon.
Statistical analysis
All statistical analysis was performed with SPSS (Statistical Package for the Social Sciences, version 27.0, IBM Corporation, Armonk, NY, USA). Probability values <0.05 were considered statistically significant. For descriptive data means (standard deviations, SD), frequencies, and medians [interquartile ranges (IQR) and maximum and minimum values] were calculated.
To decide if the participants adhered to the rehabilitation protocol, a cut-off was set at performing the exercises more than 5 days per week, at least five sessions per day, and using the splint more than 5 nights during a week.
For the multivariate logistic regression analyses, a dichotomised QuickDASH score (<30/≥30) was used as the dependent variable. Firstly, the univariate associations with QuickDASH were evaluated for the explanatory variables age (<50/≥50 years), sex (men/women), fracture type (non-displaced/displaced), hand injured (non-dominant/dominant), number of fingers injured, number of days in a cast, pain at rest at cast removal (VAS score) and fear of movement at cast removal (Tampa score). Secondly, the explanatory variables (in four categories) were entered in the final logistic model; demographics (age and sex), structural severity of the fracture (fracture type, hand injured, number of fingers injured) immobilisation time (number of days in cast) and perceived factors at cast removal (pain and fear of movement). The odds ratio and 95% confidence interval, the Nagelkerke R Square, and p-value were calculated.
Results
In , the characteristics of the 86 participants (58 women; 67%) with a mean age of 49 years (SD 18) are shown. There were 11 participants that had 2 fingers fractured (in total 97 P1 fractures) and 53% of these were non-displaced. No patients required any surgical fixation or corrective osteotomy. The participants’ dominant hand was more commonly injured (56%), a majority had a fracture on their little finger (63%) and the most common cause of injury was falling (54%). The average immobilisation time was 19 days (SD 4). On the first visit to the rehabilitation unit, when the cast was removed, 34 participants (35%) perceived pain at rest (VAS score >10) and 23 participants (28%) perceived high fear of movement (TAMPA score ≥37).
Table 1. Demographics and clinical characteristics of participants with non-surgically treated proximal phalangeal fractures (n = 86).
The mean number of visits to the rehabilitation unit from cast removal to follow-up at 6 weeks was 2.3 (SD 0.8) with a minimum of 1 and a maximum of 4 visits. In total, there were 2.6 (SD 1.3) visits, when including those that were made after the 6 weeks follow-up. In addition, 3 patients were also referred back to the hand surgeon at 8 weeks after the injury and 1 patient at 12 weeks for an additional assessment due to suspected instability. They all had an X-ray and a stability test and were found to be stable and healed.
Two patients failed to provide the exercise and splint diary, and consequently, the adherence rates to exercises and splint regime were calculated with 84 participants instead of 86. The first week after cast removal, 82% of the participants adhered to the exercise regime (>5 days and ≥5 sessions per day), and in the second week, the adherence rate was 63%. The splint was used by 62% of the participants for more than 5 nights the first week after cast removal and 7% did not use the night splint at all. In the second week, the night splint was used by 64% (>5 nights) and 16% were not using it at all.
At 6 weeks follow-up, the mean TAM was 207° (SD 31), TAM% 84% (SD 11) and the median PIP-joint deficit was 5° (IQR 0–15) (see ). The median VAS score of pain intensity in activities was 16.5 (IQR 7–37) and lower VAS levels were found at rest (median score 3) and after activities (median score 9). The mean grip strength was 66% (SD 25) compared to the uninjured side. The median value of disability of the upper extremity, the QuickDASH score, was 20 (IQR 11–30), and 50% of the participants had a score below 20 (low disability) and 28% had a score of 30 or more (high disability). At the follow-up at 6 weeks, fear of movement and self-efficacy were also rated. The median TAMPA score was 31 (IQR 26–36) and 20% of the participants had a score ≥37 (high fear of movement). The median GSE score (self-efficacy) was 31 (29–35) and 69% had a high self-efficacy (GSE score ≥30).
Table 2. Clinical and patient reported outcome at 6 weeks follow-up after non-surgically treated phalangeal fractures (n = 86).
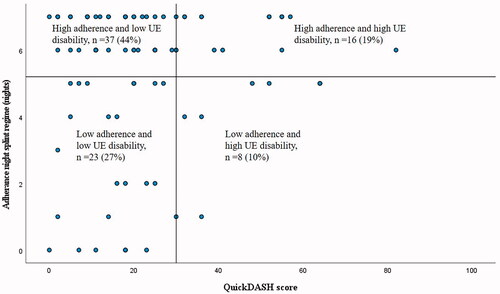
The largest group of participants adhered to exercise (56%) and a night splint regime (44%) during the first week after cast removal and perceived low upper extremity disability (QuickDASH score <30) at 6 weeks of follow-up (see and ). Some participants adhered to exercise (26%) and night splint regime (19%) and yet perceived high upper extremity disability (QuickDASH score ≥30). Of those with less adherence, the majority of patients belonged to the group that perceived low disability at 6 weeks.
Figure 3. Adherence to exercise regime (number of days with completed sessions of exercises) the first week after cast removal plotted against the upper extremity (UE) disability at 6 weeks follow-up. The cut-off for high adherence was performance of exercises more than 5 days at least five sessions a day and the cut-off for high disability was set to a QuickDASH score ≥30.
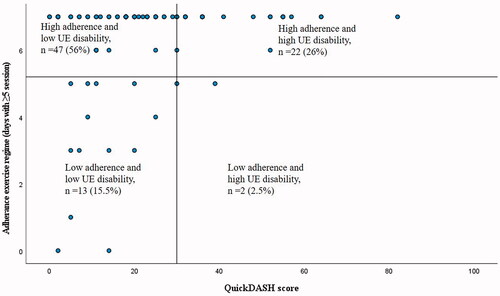
Figure 4. Adherence to night splint regime (number of nights the splint was used) the first week after cast removal plotted against upper extremity disability at 6 weeks follow-up. The cut-off for high adherence was set to splint use more than 5 nights and the cut-off for high disability was set to a QuickDASH score ≥30.
In the univariate logistic regression analyses () and the final logistic regression model (), the strongest associated factors with high upper extremity disability (QuickDASH score ≥30) 6 weeks following a P1 fracture were days in cast and fear of movement. In the final model, days in cast were the only significant predictor (Odds ratio 1.429, 95% CI 1.110–1.840, p-value 0.006). The final model had a Nagelkerke R Square value of 0.459 (p-value <0.001), and factors of Demographics (being ≥50 years and female) and Structural severity of the fracture (having a displaced fracture and the dominant hand injured) added 0.094 to the explanatory value, Immobilisation time (days in cast) added most (0.274) and Perceived factors at cast removal (pain at rest and fear of movement) another 0.091.
Table 3. Univariate logistic regression analyses of factors associated to upper extremity disability (QuickDASH score ≥30) after non-surgically treated proximal phalangeal fractures (n = 86).
Table 4. Logistic regression model of factors associated to upper extremity disability (QuickDASH score ≥30) after non-surgically treated proximal phalangeal fractures (n = 86).
Of those participants who had an immobilisation time of <15 days in casts, no one had a QuickDASH score ≥30, only one had a score >20 and one had a high fear of movement (Tampa score ≥37).
Discussion
The results of the present study showed that the majority of patients with 1 or 2 proximal non-surgically treated phalangeal fractures had regained most of their active joint motion in the injured finger by 6 weeks and reported that they had adhered to the rehabilitation protocol. However, just over a quarter of the participants still perceived high upper extremity disability in daily activities. High upper extremity disability was most influenced by being more days in cast and perceiving fear of movement. Being older than 50 years and female, having a displaced fracture in the dominant hand, and perceiving pain at rest also contributed to the final logistic regression model (explanatory value of 0.46).
The participants in the current study had regained good joint motion in the injured finger/fingers (mean TAM 207°/84%, median PIP-joint deficits 5°) and pain levels were low (median VAS <20) at 6 weeks, which is an earlier time point for evaluation than in most previous studies of non-surgically treated P1 fractures [Citation10–12]. The study of Franz et al. [Citation13] reported a mean TAM of 189° at 4 weeks and a mean TAM of 245° at 12 weeks follow-up. Byrne et al. [Citation14] found a more favourable outcome (mean TAM 253° and median PIP-joint extension deficits 4°) at a mean of 7 weeks after injury. However, their patients were followed in a range of 3–15 weeks until joint motion deficits were overcome or gains had plateaued in rehabilitation. As the time points of follow-up differ, it is difficult to fully compare the previous results to our study. In surgically treated phalangeal fractures, it has been shown that most of the improvement occurs within 6 weeks [Citation40], indicating that range of motion can be regained early after a P1 fracture in accordance with the current study.
Even though the most range of motion was recovered after the P1 fracture, grip strength had not returned to normal 6 weeks after the injury. This could probably be explained by the short follow-up period, where patients, according to the protocol, had not been allowed to fully load their hands in training or daily activities. Furthermore, about 30% of the participants perceived high upper extremity disability, measured with QuickDASH, by 6 weeks. Therefore, in clinical practice, even if most patients have an early recovery of hand function following a P1 fracture, there is still a group that may need continued rehabilitation after 6 weeks to regain normal ability to perform daily activities.
In the final logistic regression model, the explanatory value of days in cast was larger than all other included and added variables. Our results also showed that most participants that were immobilised for <15 days perceived low upper extremity disability, suggesting that outcomes can be improved when days in cast are reduced. The length of the immobilisation period is therefore an important factor to consider for attaining early recovery after a P1 fracture. This was also found in a study by Miller et al. [Citation41] evaluating surgically treated P1 fractures, where time to start active exercise was the most important predictor for active range of motion at 6 weeks post-operatively. An interesting area for further studies would be to investigate what factors influence the length of immobilisation time after P1 fracture(s) to reduce disability at 6 weeks.
Moreover, fear of movement at cast removal was the second-highest predictor of perceiving upper extremity disability. Fear of movement accounted for a larger proportion of the explanatory value in the final model than the structural severity of the fracture. Fear of movement has also been found to be an important predictor of upper extremity disability in hand injuries and may result in protective behaviour [Citation20,Citation21,Citation42]. In our study, the proportion of participants with a fear of movement only slightly decreased from cast removal until follow-up at 6 weeks (from a quarter to a fifth). Thus, fear of movement changed very little, despite 3 to 4 weeks of rehabilitation after cast removal. Our results also suggest that limiting immobilisation and starting early motion within 15 days can reduce fear of movement.
In the studies by Franz et al. [Citation13] and Byrne et al. [Citation14], only the MCP joints were immobilised and IP-joint exercises could therefore begin directly after the injury. This may be preferable for preventing fear of movement and worse outcomes. However, as these studies did not report levels of upper extremity disability or fear of movement, it is not known if hand function actually was transferred into activity performance in the usual environment. To leave the IP joints free may also increase the risk for progression to surgery, which was the case for 4 patients in the study by Franz et al. [Citation13] and 3 patients in the study by Byrne et al. [Citation14] compared to none in our study. Moreover, special casts/splints also require specialised competence and staff accessibility if pressure or discomfort occurs. The thermoplastic splint in the study by Byrne et al. [Citation14] was made by a hand therapist and more visits to a rehabilitation unit (5.3 visits) were seen compared to our study (2.6 visits); a factor that should be considered in view of health economy and resources. Thus, cast immobilisation may have some advantages but should be minimised and active movements ought to start early after injury. It may also be suggested that if cast immobilisation is used directly after the injury, it could be replaced with a splint that only immobilises the MCP joint to enable the early commencement of active movements in the wrist and IP joints.
In the current study, patients were instructed to report exercise and night splint use in a diary. Although this method may have limitations, such as inaccurate reporting and reluctance to report non-adherence, it is a commonly used method to measure adherence. Moreover, adherence diaries have shown moderately high validity and acceptability across limited populations and have the advantage of minimising memory biases due to daily reporting [Citation43,Citation44]. Adherence implies an ‘active, voluntary and collaborative involvement by the patient in a mutually acceptable course of behaviour to produce a preventative or therapeutic result’ [Citation45]. However, adherence is not a clear-cut issue. The number of completed exercise sessions per day may not reflect the quality of each exercise or the number of repetitions performed each session. Individual needs of exercise sessions may also vary depending on initial range of motion status or progression on daily basis. To treat adherence as a dichotomous variable may also be problematic since non-adherence or low vs. high adherence may be difficult to define. Nevertheless, even though the adherence rate is difficult to measure it is important knowledge for clinicians to understand the outcome relative to the actual performance of the rehabilitation protocol.
Based on our cut-off for low vs. high adherence most patients reported high adherence to the exercise regime the first 2 weeks after cast removal, but less participants adhered to the regime the second week compared to the first (60 vs. 80%). Reasons for the decrease may have been difficulties to adhere to the intense exercise protocol for more than one week. Also, the participants may have regained sufficient range of motion enabling the use of the hand in daily activities. Moreover, a narrow majority, adhered to the night splint regime in the first and second week after cast removal. The patients were informed that the aim of the splint was to protect the fracture and facilitate MCP-joint flexion and PIP-joint extension. However, non-adherence might have been due to the fact that a night splint immobilise the hand which may result in discomfort, difficulty sleeping, and a sense of stiffness in the morning. Furthermore, some participants may only have used the splint part of the night and reported this as non-use.
A majority of our patients adhered to the rehabilitation protocol and perceived low upper extremity disability by 6 weeks. Nevertheless, high adherence was also found in a group of participants with high upper extremity disability. These participants were characterised by being immobilised longer time and perceiving fear of movement. As pointed out above, immobilisation time should be minimised to avoid a poor outcome. Also, risk factors, such as pain at cast removal, being female, higher age, and a displaced fracture in the dominant hand should be identified early on to be able to provide extra support and targeted interventions. Interestingly, a majority of patients with low adherence to the rehabilitation protocol during the first week perceived low disability at 6 weeks. This group was too small in the current study (n = 13) for further subgroup analyses. We can therefore only speculate on which factors contributed to the favourable outcome. A possible reason could be that range of motion improved very quickly, which did not motivate more than 5 exercise sessions per day. Hand function may also have been regained in movements in usual activities. In future studies, adherence scales including predetermined response options for non-adherence would enable a deeper understanding of this subgroup.
Clinical implications
A standardised rehabilitation protocol works well for most patients, but the therapist must always see each patient's individual needs and adapt information and treatment accordingly. The strongest predictor of poor outcome at 6 weeks was longer immobilisation time, suggesting that days in cast and delayed motion should be minimised after a P1 fracture. Fear of movement also contributed to upper extremity disability. Clinicians should therefore pay attention to the fear of movement and be aware that immobilisation may result in the continued protection of the injured hand. Other factors, such as pain, age, and dominant hand injured, should also be taken into account in a patient-centred approach. Screening questions for fear of movement or pain may provide useful information. Adequate and reassuring information about recovery and practical guidance to patients on how to deal with challenges in daily activities may facilitate early use of the injured hand and prevent disability.
Strengths and limitations
A strength of the present study was that care was taken to standardise the test situation and an experienced physiotherapist, other than the treating therapist, performed all the assessments and received the completed diaries. The risk of influencing the patients in any direction was thereby reduced.
The sample size in the present study was sufficiently large to evaluate the associations of interest according to events per variable (EPV) of 10 which is commonly used as a ‘rule of thumb’ in medical literature as the lower limit for developing prediction models with binary outcome. However, for weak associations, a larger study sample would have been required. Other factors included in the present study may also be of importance, such as social background, occupational situation, and family support. In addition, factors important for short-term results after a P1 fracture were analysed in this study, which may not be extrapolated for long-term outcomes. Furthermore, the patients were removed from their cast at different time points and adherence was therefore investigated the first and second week after cast removal. The cut-offs for adherence were arbitrarily set, but based on clinical experience on the frequency of training and splint use expected after a finger fracture.
Conclusion
Most patients regain early satisfactory hand function, but a quarter still perceives high upper extremity disability. Longer immobilisation time in particular and fear of movement are important factors that may negatively affect early outcomes after a proximal phalangeal fracture.
Supplementary_material.docx
Download MS Word (177.9 KB)Acknowledgements
The authors are grateful to the individuals who volunteered to participate. We would also like to thank medical statistician Anna Åkesson, Department of Medical Statistics and Epidemiology at Skåne University Hospital, for statistical support.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Feehan LM, Sheps SB. Incidence and demographics of hand fractures in British Columbia, Canada: a population-based study. J Hand Surg Am. 2006;31(7):1068–1074.
- Verver D, Timmermans L, Klaassen RA, et al. Treatment of extra-articular proximal and Middle phalangeal fractures of the hand: a systematic review. Strategies Trauma Limb Reconstr. 2017;12(2):63–76.
- Held M, Jordaan P, Laubscher M, et al. Conservative treatment of fractures of the proximal phalanx: an option even for unstable fracture patterns. Hand Surg. 2013;18(2):229–234.
- LaStayo PC, Winters KM, Hardy M. Fracture healing: bone healing, fracture management, and current concepts related to the hand. J Hand Ther. 2003;16(2):81–93.
- Logters TT, Lee HH, Gehrmann S, et al. Proximal phalanx fracture management. Hand. 2018;13(4):376–383.
- Oetgen ME, Dodds SD. Non-operative treatment of common finger injuries. Curr Rev Musculoskelet Med. 2008;1(2):97–102.
- World Health Organization. International classification of functioning, disability and health; 2001. Available from: http://www.who.int/classifications/icf/en
- Baldwin PC, Wolf JM. Outcomes of hand fracture treatments. Hand Clin. 2013;29(4):621–630.
- Hardy MA. Principles of metacarpal and phalangeal fracture management: a review of rehabilitation concepts. J Orthop Sports Phys Ther. 2004;34(12):781–799.
- Figl M, Weninger P, Hofbauer M, et al. Results of dynamic treatment of fractures of the proximal phalanx of the hand. J Trauma. 2011;70(4):852–856.
- Singh J, Jain K, Mruthyunjaya Ravishankar R, et al. Outcome of closed proximal phalangeal fractures of the hand. Indian J Orthop. 2011;45:432–438.
- Rajesh G, Ip WY, Chow SP, et al. Dynamic treatment for proximal phalangeal fracture of the hand. J Orthop Surg. 2007;15(2):211–215.
- Franz T, von Wartburg U, Schibli-Beer S, et al. Extra-articular fractures of the proximal phalanges of the fingers: a comparison of 2 methods of functional, conservative treatment. J Hand Surg Am. 2012;37(5):889–898.
- Byrne B, Jacques A, Gurfinkel R. Non-surgical management of isolated proximal phalangeal fractures with immediate mobilization. J Hand Surg Eur Vol. 2020;45(2):126–130.
- de Putter CE, Selles RW, Polinder S, et al. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
- Rosberg HE, Carlsson KS, Dahlin LB. Prospective study of patients with injuries to the hand and forearm: costs, function, and general health. Scand J Plast Reconstr Surg Hand Surg. 2005;39(6):360–369.
- Moisello C, Bove M, Huber R, et al. Short-term limb immobilization affects motor performance. J Mot Behav. 2008;40(2):165–176.
- Hamasaki T, Pelletier R, Bourbonnais D, et al. Pain-related psychological issues in hand therapy. J Hand Ther. 2018;31(2):215–226.
- Keogh E, Book K, Thomas J, et al. Predicting pain and disability in patients with hand fractures: comparing pain anxiety, anxiety sensitivity and pain catastrophizing. Eur J Pain. 2010;14(4):446–451.
- Das De S, Vranceanu AM, Ring DC. Contribution of kinesophobia and catastrophic thinking to upper-extremity-specific disability. J Bone Joint Surg Am. 2013;95(1):76–81.
- Tuna Z, Oskay D. Fear of movement and its effects on hand function after tendon repair. Hand Surg Rehabil. 2018;37(4):247–251.
- Ellis B, Bruton A. A study to compare the reliability of composite finger flexion with goniometry for measurement of range of motion in the hand. Clin Rehabil. 2002;16(5):562–570.
- National Quality Registry for Hand Surgery in Sweden. National manual for measuring motion and strength in the elbow, forearm and hand. Available from: https://hakir.se/national-manual
- Hume MC, Gellman H, McKellop H, et al. Functional range of motion of the joints of the hand. J Hand Surg. 1990;15(2):240–243.
- Huskisson EC. Measurement of pain. Lancet. 1974;304(7889):1127–1131.
- Mathiowetz V, Weber K, Volland G, et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222–226.
- Hudak PL, Amadio PC, Bombardier C, et al. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602–608.
- Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44.
- Aasheim T, Finsen V. The DASH and the QuickDASH instruments. Normative values in the general population in Norway. J Hand Surg Eur Vol. 2014;39(2):140–144.
- Hunsaker FG, Cioffi DA, Amadio PC, et al. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208–215.
- Beaton DE, Davis AM, Hudak P, et al. The DASH (disabilities of the arm, shoulder and hand) outcome measure: what do we know about it now? British J Hand Ther. 2001;6(4):109–118.
- Lundberg M, Rosengren J. Kinesiofobi: teori och tillämpning. Lund: Studentlitteratur; 2008.
- Miller RP, Kori SH, Todd DD. The Tampa Scale: a measure of Kinesiophobia. Clin J Pain. 1991;7(1):51.
- Lundberg MKE, Styf J, Carlsson SG. A psychometric evaluation of the Swedish version of the Tampa Scale for kinesiophobia – from a physiotherapeutic perspective. Physiother Theory Pract. 2004;20(2):121–133.
- Vlaeyen JW, Kole-Snijders AM, Boeren RG, et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62(3):363–372.
- Schwarzer R, Jerusalem M. Self-efficacy measurement and generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: a user's portfolio causal and control beliefs. Windsor: NFER-NELSON; 1995.
- Koskinen-Hagman M, Schwarzer R, Jerusalem M. Swedish version of the general self-efficacy scale; 1999. Available from: http://userpage.fu-berlin.de/*health/swedish.htm
- Love J, Moore CD, Hensing G. Validation of the Swedish translation of the general self-efficacy scale. Qual Life Res. 2012;21(7):1249–1253.
- Scholz U. Is general self-efficacy a universal construct? Psychometric findings from 25 countries. EJPA. 2002;18:242–251.
- Miller L, Ada L, Crosbie J, et al. Pattern of recovery after open reduction and internal fixation of proximal phalangeal fractures in the finger: a prospective longitudinal study. J Hand Surg Eur Vol. 2017;42(2):137–143.
- Miller LG, Ada L, Crosbie J, et al. Time to commencement of active exercise predicts total active range of motion 6 weeks after proximal phalanx fracture fixation: a retrospective review. Hand Therapy. 2017;22(2):73–78.
- Wilkens SC, Lans J, Bargon CA, et al. Hand posturing is a nonverbal indicator of catastrophic thinking for finger, hand, or wrist injury. Clin Orthop Relat Res. 2018;476(4):706–713.
- Essery R, Geraghty AW, Kirby S, et al. Predictors of adherence to home-based physical therapies: a systematic review. Disabil Rehabil. 2017;39(6):519–534.
- Frost R, Levati S, McClurg D, et al. What adherence measures should be used in trials of home-based rehabilitation interventions? A systematic review of the validity, reliability, and acceptability of measures. Arch Phys Med Rehabil. 2017;98(6):1241–1256.e45.
- Meichenbaum D, Turk DC. Facilitating treatment adherence: a practitioner's guidebook. New York (NY): Plenum Press; 1987.
