Abstract
Objective: The aim of this study was to assess the risk of serious adverse effects after radiotherapy (RT) with curative intention and radical prostatectomy (RP).
Materials and methods: Men who were diagnosed with prostate cancer between 1997 and 2012 and underwent curative treatment were selected from the Prostate Cancer data Base Sweden. For each included man, five prostate cancer-free controls, matched for birth year and county of residency, were randomly selected. In total, 12,534 men underwent RT, 24,886 underwent RP and 186,624 were controls. Adverse effects were defined according to surgical and diagnostic codes in the National Patient Registry. The relative risk (RR) of adverse effects up to 12 years after treatment was compared to controls and the risk was subsequently compared between RT and RP in multivariable analyses.
Results: Men with intermediate- and localized high-risk cancer who underwent curative treatment had an increased risk of adverse effects during the full study period compared to controls: the RR of undergoing a procedures after RT was 2.64 [95% confidence interval (CI) 2.56–2.73] and after RP 2.05 (95% CI 2.00–2.10). The risk remained elevated 10–12 years after treatment. For all risk categories of prostate cancer, the risk of surgical procedures for urinary incontinence was higher after RP (RR 23.64, 95% CI 11.71–47.74), whereas risk of other procedures on the lower urinary tract and gastrointestinal tract or abdominal wall was higher after RT (RR 1.67, 95% CI 1.44–1.94, and RR 1.86, 95% CI 1.70–2.02, respectively).
Conclusion: The risk of serious adverse effects after curative treatment for prostate cancer remained significantly elevated up to 12 years after treatment.
Introduction
Men with low- and intermediate-risk cancer who have been treated with curative intent have a 10 year prostate-cancer specific mortality of less than 5% [Citation1], so long-term functional outcomes are of paramount importance for these men.
Several large studies have directly compared outcomes after radiotherapy (RT) and radical prostatectomy (RP) in the intermediate term. In a register-based study in Canada of 30,000 men who underwent RT or RP for localized prostate cancer, the risk of hospital admissions up to 5 years after treatment was higher after RT than RP for rectal and anal procedures and open surgical procedures, whereas the risk of surgical procedures for urological conditions was higher after RP [Citation2]. In a report from the Prostate Cancer Outcomes Study (PCOS) in the USA, urinary incontinence and erectile dysfunction were more common after RP than after RT, whereas bowel symptoms were more frequent 5 years after RT but after 15 years was the risk similar after RT and RP [Citation3].
To the authors’ knowledge, no studies have reported the frequency of long-term adverse effects after RT and RP in comparison with the background population. Furthermore, direct comparisons of RT and RP must be interpreted with caution as there is a selection bias towards younger and healthier men with fewer adverse cancer features for RP [Citation4]. Furthermore, to assess the risk of adverse effects after treatment, attention must be paid to the prevalence of urinary and gastrointestinal symptoms in the background population, which increases with higher age [Citation5].
The aim of this study was to assess the risk of long-term adverse effects after RT and RP for men who had been treated for localized or locally advanced prostate cancer compared with the background population, and subsequently to compare risk after RT and RP using data in nationwide, population-based health care registers and demographic databases in Sweden.
Materials and methods
Study population and data collection
The National Prostate Cancer Register (NPCR) of Sweden captures 98% of all newly diagnosed prostate cancer cases in the Swedish Cancer Registry, to which registration is compulsory and mandated by law [Citation6,Citation7]. NPCR data include date of diagnosis, age, tumor stage, tumor differentiation, serum level of prostate-specific antigen (PSA) and primary treatment. The Prostate Cancer data Base Sweden (PCBaSe) 3.0 has recently been described in detail [Citation8]. In brief, by record linkage using the unique Swedish personal identity number, information for men with prostate cancer in the NPCR and five prostate-cancer free men, randomly selected from groups of men in the background population matched for birth year and county of residence, was obtained from a number of national healthcare registries and demographic databases.
This study included men diagnosed with prostate cancer between 1997 and 2012 who had received curative RT or RP within 1 year after the date of diagnosis. Men who received salvage RT or RP ended their follow-up time at the date of secondary treatment and men with stage T4, N1 or M1 disease or serum PSA above 50 ng/ml at diagnosis were excluded. In a modification of the National Comprehensive Cancer Network (NCCN) categorization, the following risk categories were defined: low risk (local clinical stage T1–2, Gleason score ≤6 and PSA <10 ng/ml), intermediate risk (T1–2, Gleason score 7 and/or PSA 10 to <20 ng/ml), localized high risk (T1–2 and/or Gleason score 8–10 and/or PSA 20 to <50 ng/ml) and locally advanced (T3). The intermediate- and localized high-risk categories were merged into one group.
Date of treatment, comorbidity and socioeconomic factors
The National Patient Registry includes diagnoses and procedures from inpatient and outpatient care that have been found to be 85–95% accurate [Citation9]. Data from the National Patient Registry were used to determine date of treatment and retrieved diagnoses [according to International Classification of Diseases (ICD) 9 or 10] and procedures [according to the Nordic Medico-Statistical Committee (NOMESCO) classification of surgical procedures] as measures of adverse effects after treatment. The Charlson Comorbidity Index (CCI) was calculated as previously described [Citation10,Citation11]. Data on socioeconomic factors including marital status and educational level were retrieved for each subject from the Longitudinal Integration Database for Health Insurance and Labor Market Studies [Citation12]. The levels of education were low (compulsory school, 9 years), intermediate (high school, 10–12 years) and high (college, >12 years). Data on type of RT were combined from NPCR and RETRORAD, an audit that collected information on type of RT, treatment time, total dose and fractionation directly from the RT verification/oncology information systems and local databases in oncology departments in Sweden [Citation13].
Classification of diagnostic and surgical procedures
Discharge diagnoses and surgical procedures that indicated adverse effects to treatment were retrieved from the National Patient Registry. The diagnoses were classified into four domains: (1) urinary incontinence; (2) storage lower urinary tract symptoms (LUTS); (3) obstructive LUTS; and (4) gastrointestinal diagnoses. Surgical procedures were also classified into four domains: (1) procedures for urinary incontinence; (2) procedures on the lower urinary tract; (3) procedures on the ureters, renal pelvis, kidneys, male genital organs and reoperations; and (4) procedures on the gastrointestinal tract and abdominal wall. The most common diagnoses and procedures are listed in supplementary Tables S1 and S2.
Statistical methods
Incidence rate ratios, as a measure of relative risk (RR), of diagnoses and procedures indicating adverse effects were calculated for each 3 year interval up to 12 years after primary treatment. The main analysis focused on men with intermediate- and high-risk cancer as this was the most balanced group in terms of proportion of men who underwent RT or RP. In multivariable analysis including treatment year, age, CCI, educational level, serum level of PSA, clinical T stage and biopsy Gleason score, RR was estimated by incidence rate ratios from Poisson regression models in 3 year intervals after treatment [Citation14]. To avoid including the same event more than once, a second identical diagnosis or procedure was ignored within 2 months after the first event. This period was also excluded from the time at risk in all analyses. Time was introduced as an offset in the models to account for the time men spent at risk in each interval.
All statistical tests were two sided and all analyses were performed using R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) software. The research ethics review board at Umeå University Hospital approved the study.
Results
In total, 12,534 men underwent RT, 24,886 men underwent RP and 186,624 prostate cancer-free men were controls. Baseline characteristics for these men including risk category, comorbidity and demographics are presented in . Men treated with RT were older and had more comorbidities than men who underwent RP. Locally advanced cancer was more common among men who received RT (32%) compared to RP (8%), whereas a lower proportion of men treated with RT (22%) had low-risk cancer than men treated with RP (43%). Type of RP and RT per calendar period is demonstrated in supplementary Table S3. The number of RT and RP performed annually increased during the study period and the use of robot-assisted RP increased more than other treatments.
Table 1. Baseline characteristics of men diagnosed with prostate cancer (PCa) between 1997 and 2012 who received radiotherapy (RT)Table Footnotea or a radical prostatectomy (RP)Table Footnotea and their matched PCa-free men in the Prostate Cancer data Base (PCBaSe) 3.0.
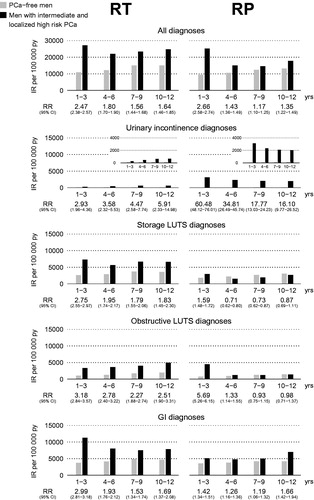
To analyze outcome in men with similar risk profile and groups of similar size, the main analyses were performed on men with intermediate- and localized high-risk cancer. shows the risk for diagnoses over time. The risk of all diagnoses combined remained modestly but significantly elevated after both RT and RP up to 12 years after treatment, 10–12 years after RT [RR 1.64, 95% confidence interval (CI) 1.46–1.85] and after RP (RR 1.35, 95% CI 1.22–1.49). The risk of urinary incontinence was strongly elevated during all periods after RP, and remained high after 10–12 years (RR 16.10, 95% CI 9.77–26.52). Urinary incontinence was diagnosed in 1274 out of 24,886 men (5%) after RP and in 167 out of 12,534 men (1%) after RT. Three years after RP, the risk of storage and obstructive LUTS was similar to the risk in the background population, whereas corresponding risks remained elevated after RT in all time intervals after treatment.
Figure 1. Relative risk of diagnoses indicating adverse effects up to 12 years after treatment for intermediate- and localized high-risk prostate cancer (PCa) vs age-matched prostate cancer-free men. RT: radiotherapy; RP: radical prostatectomy; IR: incidence rate; py: patient-years; RR: relative risk; CI: confidence interval; LUTS: lower urinary tract symptoms; GI: gastrointestinal.
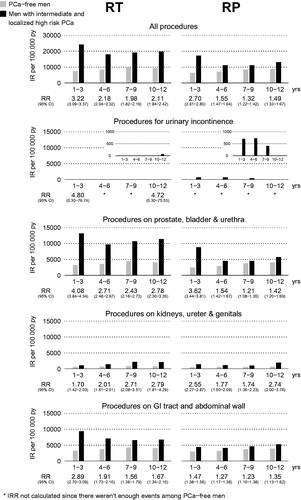
shows the risk of all procedures and the specific procedures within the four selected domains. During the entire study period, the risk for all procedures was elevated after RT and RP, at 10–12 years after RT (RR 2.11, 95% CI 1.84–2.42) and after RP (RR 1.49, 95% CI 1.33–1.67). The risk of adverse effects in men with low-risk and locally advanced high-risk prostate cancer (see supplementary Figures S1a,b and S2a,b) was similar to that in men with intermediate- and high-risk cancer.
Figure 2. Relative risk of procedures indicating adverse effects up to 12 years after treatment for intermediate- and localized high-risk prostate cancer (PCa) vs age-matched prostate cancer-free men. RT: radiotherapy; RP: radical prostatectomy; IR: incidence rate; py: patient-years; RR: relative risk; CI: confidence interval; GI: gastrointestinal; IRR: incidence risk ratio.
The risk for adverse effects after RP and RT was subsequently compared in multivariable analyses of men in all prostate cancer risk categories (). The risk of all diagnoses during the full study period was higher after RT than RP (RR 1.19, 95% CI 1.10–1.28). During the first 3 years after treatment, the risk of all diagnoses was similar after RT and RP, but with longer follow-up, risk became higher after RT (RT vs RP at 10–12 years: RR 1.57, 95% CI 1.10–2.22). During the full study period, the risk of a diagnosis indicative of storage LUTS was higher after RT than after RP, whereas the risk of obstructive LUTS increased over time for RT versus RP, to an RR of 4.20 at 10–12 years (95% CI 1.84–9.62).
Table 2. Relative risks (RRs) of diagnoses and procedures indicating an adverse effect of treatment for men in all risk categories of prostate cancer (PCa) who received radiotherapy compared to men who underwent a prostatectomy.
For all procedures combined, risk was higher for RT than RP during all periods (RR 1.49, 95% CI 1.39–1.59), whereas the risk of a procedure for urinary incontinence was much higher after RP than after RT (RR 23.64, 95% CI 11.71–47.74). A surgical procedure for urinary incontinence was performed in 572 out of 24,886 men (2%) after RP and in 10 out of 12,534 men (0.1%) after RT. The risk of a procedure on the lower urinary tract was higher after RT than after RP (RR 1.67. 95% CI 1.44–1.94), as was the risk of a procedure on the gastrointestinal tract and abdominal wall (RR 1.86, 95% CI 1.70–2.02). The most common procedure on the gastrointestinal tract after RT was endoscopy; specifically, 1478 out of 12,534 men (12%) underwent colonoscopy, 6% flexible sigmoidoscopy, 10% proctoscopy and 4% anoscopy. The risks of diagnoses and procedures between men who received RT and RP in low-risk categories were essentially similar.
Discussion
In this nationwide, population-based study, the risk of all diagnoses and procedures indicating a serious adverse effect of treatment was modestly but significantly increased after RT and RP compared to matched prostate cancer-free men, and this increase remained up to 12 years after treatment. There was a higher risk after RT compared to RP for all diagnoses and procedures combined. There was a higher risk after RT compared to RP for all diagnoses and procedures combined. Specifically, the risk of LUTS and gastrointestinal diagnoses and procedures was higher after RT. However, the risk of urinary incontinence was much higher after RP than after RT.
The strengths of this study include the nationwide, population-based cohort based on the 98% capture to NPCR, the use of matched prostate cancer-free men and the analysis of risk in time intervals [Citation15]. The follow-up was longer than in most previous studies that have reported short-term (1–3 year) [Citation16,Citation17] or intermediate-term (4–5 year) outcomes [Citation18,Citation19]. Resnick and Penson published data on urinary incontinence and erectile dysfunction at 2, 5 and 15 years after RT and RP [Citation20], but data on other adverse effects to treatment are scarce. Furthermore, the present study included data from all public healthcare providers in Sweden, including virtually all treatments performed between 1997 and 2012 and discharge diagnoses in the National Patient Registry, which has been documented to be 85–95% accurate [Citation9]. The present study also used information on comorbidity and socioeconomic factors from healthcare registries and demographic databases. Furthermore, to account for the incidence of diagnoses and procedures in the background population, the risk was calculated compared to prostate cancer-free men, and the risk after RT and RP was subsequently compared.
There are some limitations to this study. The National Patient Registry captures almost all inpatient episodes but the capture of outpatient episodes is lower, approximately 80%, mainly because of inadequate data from private caregivers [Citation9]. In addition, the current study did not capture adverse effects not leading to a hospital admission or a consultation, so the risk of adverse effects in some areas was underestimated as only the most serious adverse effects were captured. For example, erectile dysfunction was not captured although it is a common adverse effect after RP and RT. In a recent study, based on essentially the same population as the current study, a prescription for a 5-phosphodiesterase inhibitor was filled for 33% of men after RT and 74% after RP [Citation21]. The proportion of men with a diagnosis indicating urinary incontinence after RP was 5% in this study, whereas questionnaire data from NPCR have shown much higher proportions, with 14% of men reporting moderate incontinence and 10% reporting severe incontinence [Citation8].
The pattern of adverse effects observed after RP and RT in this study is in accordance with previous studies. Obstructive LUTS was increased during the first 3 years after RP and then decreased strongly, in line with prior reports that showed that most urethral strictures occur in the first year after RP [Citation22,Citation23]. In contrast, obstructive LUTS after RT occurred later and remained elevated up to 12 years after treatment. The risk of diagnoses and surgeries indicating storage LUTS was elevated after RT but not after RP. Accordingly, in a recent study from SEER-Medicare, interventions for bladder spasm, cystitis and hematuria were twice as common after RT than after RP [Citation24]. In another SEER study, RT was associated with an increased risk of urinary adverse effects at 10 years after treatment and thereafter [Citation25]. Two studies on health-related quality of life after RT or RP reported the strongest decline during the first year after treatment, with a plateau or some mild decline over time [Citation26,Citation27]. The risk of gastrointestinal adverse effects was somewhat higher after RT for both diagnoses and procedures up to 6 years after treatment, but thereafter the increase was similar for RT and RP. The current results on anorectal procedures were similar to those of Nam et al., who showed higher risk after RT than after RP [Citation2] but differed from the PCOS, which reported no difference in risk of urinary incontinence or bowel urgency 15 years after RT or RP [Citation3]. Furthermore, the present results are in line with those of Wallis et al., who found that for all endpoints except for urological procedures, the risk of complications was higher after RT than after RP [Citation28].
The risks of adverse effects in this study are based on results from all hospitals that treat prostate cancer in Sweden in a contemporary period; they were similar to those from other register-based large studies [Citation2,Citation23,Citation25] and are likely to be more generalizable than results from tertiary referral centers [Citation29,Citation30]. Somewhat disappointingly, risk after treatment for low-risk prostate cancer was quite similar to that for men in other risk categories.
In conclusion, the risk of all diagnoses and procedures indicating an adverse effect was modestly but significantly elevated after both RT and prostatectomy up to 12 years after treatment. The risk of urinary incontinence was much higher after RP, whereas the risk of all diagnoses and procedures, and specifically other LUTS and gastrointestinal adverse effects, was higher after RT.
Funding information
Funding was provided by the Swedish Research Council [825-2012-5047], the Swedish Cancer Society [140570], Västerbotten County Council and Lion’s Cancer Research Foundation at Umeå University. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review and approval of the manuscript.
Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Anna Bill Axelson, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, Bill Pettersson, David Robinson, Mats Andén, Jan-Erik Damber, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, Göran Ahlgren, Ola Bratt, René Blom, Lars Egevad, Calle Waller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin and Karin Hellström.
Disclosure statement
Anders Widmark worked for Sanofi as a medical advisor until 31 August 2014; he has received honoraria from Ipsen, Astellas, Janssen and Sanofi, had a consulting role for Exini, and received research funding from Galderma/Q-med and travel compensation. Pär Stattin received honoraria from AstraZeneca and Ferring.
References
- Stattin P, Carlsson S, Holmstrom B, Vickers A, Hugosson J, Lilja H, et al. Prostate cancer mortality in areas with high and low prostate cancer incidence. J Natl Cancer Inst 2014;106:dju007.
- Nam RK, Cheung P, Herschorn S, Saskin R, Su J, Klotz LH, et al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol 2014;15:223–31.
- Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013;368:436–45.
- Hoffman RM, Koyama T, Fan KH, Albertsen PC, Barry MJ, Goodman M, et al. Mortality after radical prostatectomy or external beam radiotherapy for localized prostate cancer. J Natl Cancer Inst 2013;105:711–8.
- Hakkinen JT, Hakama M, Huhtala H, Shiri R, Auvinen A, Tammela TL, et al. Impact of LUTS using bother index in DAN-PSS-1 questionnaire. Eur Urol 2007;51:473–7; discussion 7-8.
- Wallerstedt A, Tyritzis SI, Thorsteinsdottir T, Carlsson S, Stranne J, Gustafsson O, et al. Short-term results after robot-assisted laparoscopic radical prostatectomy compared to open radical prostatectomy. Eur Urol 2015;67:660–70.
- Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 2009;48:27–33.
- Van Hemelrijck M, Wigertz A, Sandin F, Garmo H, Hellstrom K, Fransson P, et al. Cohort profile: the National Prostate Cancer Register of Sweden and Prostate Cancer Data base Sweden 2.0. Int J Epidemiol 2013;42:956–67.
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450
- Berglund A, Garmo H, Tishelman C, Holmberg L, Stattin P, Lambe M. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol 2011;185:833–9.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83.
- Statistics Sweden. Longitudinal integration database for health insurance and labour market studies (LISA by Swedish acronym). http://www.scb.se. Available from: http://www.scb.se/Pages/List____257743.aspx (last accessed 23 June 2015).
- Hemelrijck MV, Garmo H, Wigertz A, Nilsson P, Stattin P. Cohort profile update: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base – a refined prostate cancer trajectory. Int J Epidemiol 2016;45:73–82.
- Rothman JR, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008.
- Tomic K, Berglund A, Robinson D, Hjalm-Eriksson M, Carlsson S, Lambe M, et al. Capture rate and representativity of the National Prostate Cancer Register of Sweden. Acta Oncol 2015;54:158–63.
- Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–61.
- Potosky AL, Legler J, Albertsen PC, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582–92.
- Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst 2009;101:888–92.
- Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, Penson DF, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2004;96:1358–67.
- Resnick MJ, Penson DF. Functional outcomes after treatment for prostate cancer. N Engl J Med 2013;368:1654.
- Plym A, Folkvaljon Y, Garmo H, Holmberg L, Johansson E, Fransson P, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med 2014;11:2100–8.
- Chen AB, D'Amico AV, Neville BA, Earle CC. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol 2006;24:5298–304.
- Elliott SP, Meng MV, Elkin EP, McAninch JW, Duchane J, Carroll PR. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol 2007;178:529–34; discussion 34.
- Jarosek SL, Virnig BA, Chu H, Elliott SP. Propensity-weighted long-term risk of urinary adverse events after prostate cancer surgery, radiation, or both. Eur Urol 2015;67:273–80.
- Kim S, Moore DF, Shih W, Lin Y, Li H, Shao YH, et al. Severe genitourinary toxicity following radiation therapy for prostate cancer – how long does it last? J Urol 2013;189:116–21.
- Miller DC, Sanda MG, Dunn RL, Montie JE, Pimentel H, Sandler HM, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol 2005;23:2772–80.
- Punnen S, Cowan JE, Chan JM, Carroll PR, Cooperberg MR. Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE registry. Eur Urol 2015;68:600–8.
- Wallis CJ, Cheung P, Herschorn S, Saskin R, Su J, Klotz LH, et al. Complications following surgery with or without radiotherapy or radiotherapy alone for prostate cancer. Br J Cancer 2015;112:977–82.
- Loeb S, Roehl KA, Helfand BT, Catalona WJ. Complications of open radical retropubic prostatectomy in potential candidates for active monitoring. Urology 2008;72:887–91.
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9.
