Abstract
Objective: Reports from cancer registries often lack clinically relevant information, which would be useful in estimating the prognosis of individual patients with urothelial carcinoma of the urinary bladder (UCB). This article presents estimates of crude probabilities of death due to UCB and the expected loss of lifetime stratified for patient characteristics.
Materials and methods: In Norway, 10,332 patients were diagnosed with UCB between 2001 and 2010. The crude probabilities of death due to UCB were estimated, stratified by gender, age and T stage, using flexible parametric survival models. Based on these models, the loss in expectation of lifetime due to UCB was also estimated for the different strata.
Results: There is large variation in the estimated crude probabilities of death due to UCB (from 0.03 to 0.76 within 10 years since diagnosis) depending on age, gender and T stage. Furthermore, the expected loss of life expectancy is more than a decade for younger patients with muscle-invasive UCB and between a few months and 5 years for nonmuscle-invasive UCB.
Conclusions: The suggested framework leads to clinically relevant prognostic risk estimates for individual patients diagnosed with UCB and the consequence in terms of loss of lifetime expectation. The published probability tables can be used in clinical praxis for risk communication.
Introduction
Reports from national and international cancer registries [Citation1–3] and public databases [Citation4,Citation5] estimate bladder cancer incidence, mortality and survival. To communicate information about prognosis, relative survival estimates are usually presented, and survival trends over time are based on 5 year relative survival estimates. For example, from the recent report from the Norwegian Cancer Registry, the 5 year relative survival estimate for male patients with cancer of the bladder, ureter or urethra (C66–C68), which is 73.1 [95% confidence interval (CI): 71.2–74.9], can be retrieved [Citation1]. The interpretation of this estimate can be misleading. This measure does not mean that, on average, 73.1% of male patients with cancer of the bladder, ureter or urethra (C66–C68) will be alive 5 years after diagnosis. Rather, it means that the observed survival in the patient group (C66–C68) is only 73.1% of the expected survival of the underlying Norwegian population.
The relative survival rates routinely published by cancer registries are usually based on net mortality, i.e. the probability of death due to cancer assuming the absence of other causes of death. This is useful for comparison of survival or the probability of death from the relevant cancer over time or across countries. However, relative survival as usually published in reports is not very informative either to the individual patient or to the treating clinician, for two reasons. First, these probabilities are not able to distinguish between the risk of dying from cancer and the risk of dying from other causes. Secondly, the published risk estimates often do not account for tumor characteristics, such as stage and grade, in addition to age and gender. Thus, both the patient and the treating clinician would benefit from a more patient-specific risk estimate communicated by a "real-world" probability of death due to cancer in the presence of other causes. This is referred to as the crude mortality or crude probability of death. This measure is also sometimes referred to as cumulative incidence [Citation6] or the absolute probability of death [Citation7]. The present article uses a method introduced by Lambert et al. [Citation8], which estimates the crude probability of death due to cancer after fitting a relative survival model to individual patient data, thus avoiding the use of cause of death information.
Although crude mortality rates are useful when aiming to determine individual cancer-related mortality as described above, this measure does not cover the impact of the individual cancer diagnosis with respect to the patient’s life expectancy. The loss in expectation of life due to cancer is estimated as the difference between the expected remaining life in the absence and presence of cancer. This quantity is of particular interest for the patient and the treating clinician, since the impact of a cancer diagnosis on the life expectancy provides additional information. The loss in expectation of life due to cancer can also be reported as a personalized measure, e.g. the estimation can be performed by incorporating additional individual variables such as gender, age and T stage. Moreover, this measure is also of public health interest because of its interpretation in terms of the impact of a cancer diagnosis in the underlying population.
In this paper, the crude probabilities of death in Norway based on patients diagnosed with urothelial carcinoma of the urinary bladder (UCB) between 2001 and 2010, including both invasive and non-invasive UCB, are estimated using data from the Cancer Registry of Norway. The crude probabilities of death will be presented for different combinations of gender, age and T stage to increase the clinical relevance. Estimates are also provided for the loss in expectation of life in UCB patients depending on age at diagnosis, stratified by gender and tumor stage.
Materials and methods
Materials
The Cancer Registry of Norway has, since 1953, registered virtually all new cancer diagnoses in Norway, and registration is compulsory by law. The registry receives information from three independent sources (clinicians, pathology laboratories and the Cause of Death Registry), which ensures completeness and high-quality data [Citation9]. Patients are identified by the unique national personal identification number which has been assigned to all newborns and residents in Norway since 1960. The present study comprises all new cases of histologically verified invasive and non-invasive UCB in the Cancer Registry of Norway diagnosed between 2001 and 2010. UCB cases were selected based on morphological codes for the transitional cell type. In total, 10,332 UCB patients were included in this study. Participants were followed until death, migration or end of follow-up on 31 December 2014, whichever came first. The total follow-up time was 56,980 person-years, with a median follow-up time of 8.1 years. The cause of death was last updated on 31 December 2013.
Morphology, T, N and M categories, and grade were defined based on the most severe diagnosis within a 4 month window from UCB diagnosis. Patients were grouped based on the available T, N and M categories, morphology and grade information, into non-invasive papillary carcinoma, low and high grade (WHO 1973 [Citation10]: TaG1G2, TaG3); non-invasive flat carcinoma (Tis); invasive carcinoma (in the connecting tissue, not muscle) (T1); and muscle-invasive carcinoma (T2–4).
Statistics
Information on T stage was missing in 33.1% (3426 out of 10,332) of the cases in this study. This incompleteness cannot be assumed to be completely random since, for example, there is a correlation between age and the proportion of reported T stages. Older patients (aged ≥80 years) had a significantly higher proportion of missing data (35.3%) than younger patients (aged ≤64 years; 32.0%) and patients diagnosed at the age of 65–79 years (32.5%) (p = 0.018, chi-squared test). Multiple imputation (mi function in Stata) was used to generate 10 imputed data sets [Citation11,Citation12], which were used for further analyses. Information about morphology, metastases, lymph-node status, survival time, status (dead or alive), gender and age was used in an ordered logistic regression to predict the T stage for patients with missing values. The method of Rubin [Citation13,Citation14] was used to retrieve the combined point estimates and confidence intervals from the results of the 10 imputed data sets.
A flexible parametric relative survival model [Citation15–17] was used, where gender, stage and age were included as categorical variables. The baseline hazard was modeled using 5 degrees of freedom (df) for the spline variables using the Stata command stpm2 [Citation18]. All variables (gender, age and T stage) were modeled as time-dependent covariates with 2 df for each time-dependent effect. Since a relative survival model was used, no cause of death information was used in the calculations. Instead, this model compares the observed survival in the bladder cancer patient group to the expected survival of the underlying Norwegian population. After fitting the model, the excess mortality rate and overall survival could be estimated for any particular covariate pattern and the crude probabilities were determined as described in Lambert et al. [Citation8]. The quantities reported are the crude probabilities of UCB-related death 1, 5 and 10 years after diagnosis together with 95% confidence intervals.
Based on the same model, the loss in expectation of lifetime due to UCB was estimated for the different subgroups (gender and T stage) by age. The loss in expectation of life due to UCB is the difference between the life expectancy in the absence of cancer and the life expectancy among the cancer patients [Citation19]. The life expectancy in the absence of cancer can be estimated from Norwegian population mortality tables. Estimation of life expectancy generally requires extrapolation of the survival function, owing to limited follow-up, since it requires all subjects to have died. It has been shown that the observed survival function can be extrapolated reliably using flexible parametric survival models within a relative survival approach [Citation20].
All statistical analyses were performed in Stata 14/MP for Windows [Citation21].
Results
Patient and tumor characteristics
In total, 10,332 cases were diagnosed with UCB (transitional cell type) in Norway between 2001 and 2010. Patient characteristics with respect to gender, vital status (UCB and other cancer-related death, alive, unknown), T stage and age are provided in . Of the UCB patients diagnosed between 2001 and 2010, 75.6% were men. For all patients (men and women combined), the median age at diagnosis was 73 years (10–90% percentiles: 57–85 years), women being about 1 year older than men. Half of the patients (50%) were diagnosed with non-invasive papillary carcinoma Ta (41% G1G2, 9% G3) and 1% with carcinoma in situ (Tis). Carcinoma invading only the lamina propria (T1) accounted for 16% and muscle-invasive carcinoma (T2–4) for 34% of all UCB cases in this study. There were 4% more muscle-invasive tumors in women than in men. Out of all patients diagnosed between 2001 and 2010, 51% had died by the end of 2014, due to either UCB (21%), or cancer (not UCB) (11%) or causes other than cancer (19%). Based on the death certificates, there were relatively more UCB-related deaths in women than in men (24% vs 20%).
Table 1. Patient characteristics: distribution of age, T stage and cause of death, by gender and in total in the underlying study population.
Crude probability of death
The crude probabilities could be calculated for any combination of age, gender and T stage. For representative purposes, the ages of 57, 73 and 85 years were chosen, corresponding to the 10%, 50% and 90% percentiles of the age distribution in the study sample. illustrates the estimated crude probabilities of death due to UCB and due to other causes for male () and female () patients with a UCB diagnosis. shows the estimated crude probabilities of death due to UCB including confidence intervals.
Figure 1. Estimated crude probabilities of death due to urothelial carcinoma of the urinary bladder (UCB) (dark gray) and due to other causes (light gray) for up to 10 years after diagnosis for any combination of gender (A: male; B: female), age (57, 73 or 85 years) and T stage (TaG1G2, TaG3, Tis, T1, T2–4).
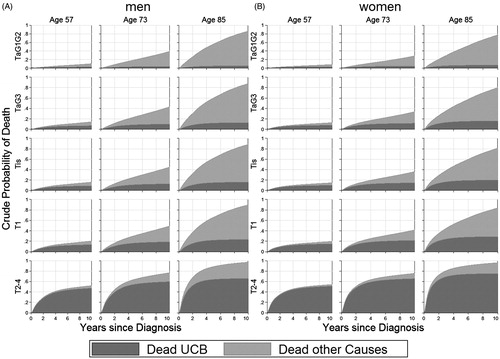
Table 2. Crude probabilities of death due to UCB (including confidence intervals) for patients with urothelial carcinoma of the urinary bladder.
The crude probabilities of death due to causes other than UCB naturally increased with age, which is illustrated by the extension of the light gray area across the columns (ages) in ). The 1 year crude probabilities of death due to UCB increased with increasing age; the risk estimates were 0.18 (95% CI 0.16–0.20), 0.27 (95% CI 0.25–0.29) and 0.38 (95% CI 0.35–0.41) for male patients with muscle-invasive UCB aged 57, 73 and 85 years, respectively. The corresponding 10 year crude probabilities of UCB-related death were 0.47 (95% CI 0.43–0.51), 0.60 (95% CI 0.57–0.63) and 0.66 (95% CI 0.63–0.69), respectively. This trend was also present for patients diagnosed with non-muscle-invasive UCB. The older the patient, the more rapid the increase in the crude probability of death after diagnosis. This is due to very few of the oldest patients surviving more than the first few years after diagnosis.
The crude probabilities of death due to UCB increased with increasing severity of the primary diagnosis (T stage). For example, the 10 year probability of UCB-related death for a 73-year-old male patient was 0.05 (95% CI 0.03–0.06) for a non-invasive low-grade papillary tumor (TaG1G2), 0.10 (95% CI 0.07–0.13) for a high-grade papillary tumor (TaG3) and 0.60 (95% CI 0.57–0.63) for muscle-invasive tumors (). The differences in the crude probabilities of death due to UCB were most pronounced between the non-muscle-invasive tumors (TaG1G2, TaG3, Tis and T1) and muscle-invasive tumors (T2–4).
The described trends were also present for female UCB patients, but the crude probabilities of UCB-related death were consequently higher for men than for women.
Loss in expectation of life
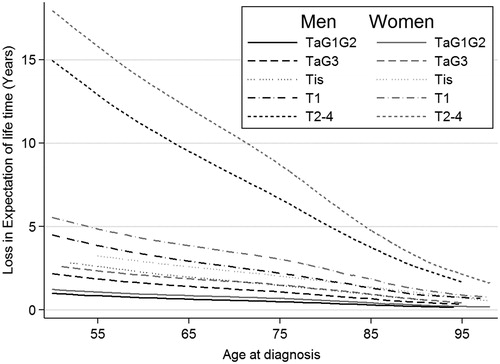
shows the estimated loss in expectation of life (in years) including confidence intervals for male and female UCB patients at the ages of 57, 73 and 85 years for all five T stages considered here (TaG1G2, TaG3, Tis, T1 and T2–4). illustrates the loss in expectation of lifetime depending on age and by T stage for male UCB patients.
Table 3. Estimated loss in expectation of lifetime including confidence intervals (in parentheses), by age, gender and T stage.
The loss in expectation of life is largest for severe T stage and young age. A 57-year-old male UCB patient has a loss in expectation of lifetime of 12.4 (95% CI 11.3–13.6) years when diagnosed with a muscle-invasive tumor. The corresponding loss in expectation of lifetime for women is larger, at 15.2 (95% CI 13.4–17.0) years. For comparison, a 57-year-old male patient diagnosed with T stage TaG1G2 has an expected reduction in lifetime of 0.8 (95% CI 0.6–1.1) years, with the corresponding reduction in females being 1.0 (95% CI 0.7–1.4) years. With the diagnosis of a muscle-invasive tumor T2–4 at the age of 85 years, the lifetime expectancy is reduced to 3.7 (95% CI 3.6–3.9) years for male and 5.2 (95% CI 4.9–5.4) years for female patients.
Discussion
This study presents crude mortality and loss in expectation of lifetime based on data on 10,322 patients from the Cancer Registry of Norway diagnosed with UCB between 2001 and 2010, stratified for gender, age and T stage. All estimates presented in this article rely on relative survival models and are thus determined independent of cause of death information.
It has been shown that the 5 year crude probability of death due to UCB for a UCB patient ranges between 0.03 and 0.74, depending on gender, age and T stage at first diagnosis. This illustrates the necessity of using the crude probability of UCB-related death as a measure and taking information on gender, age and T stage into account when aiming to describe individual risks. In contrast to cause-specific mortality, this approach is independent of the information from death certificates.
The crude probability of UCB-related death increases with increasing age. Thus, it seems that age is a major risk factor. There is also a higher risk of UCB-related death with increasing T stage at initial diagnosis. This study also showed that the increase in the crude probability of UCB-related death is larger in the first few years after diagnosis for patients diagnosed with UCB. A possible explanation for this fact is that those who die of UCB are likely to die within a few years of diagnosis.
The crude probability of death due to other causes increases with increasing age, which is simply due to a higher (not cancer-related) mortality at older ages. The observed decrease in the crude probability of death due to other causes with increasing severity is just due to the simultaneous increase in UCB-related deaths for these patients. Patients diagnosed with muscle-invasive bladder cancer are more likely to die from their cancer than patients with a non-muscle-invasive diagnosis, thus leading to fewer cancer survivors, who could die of other causes.
To the authors’ knowledge, there are no publications determining the crude probabilities of death for bladder cancer. Thus, the only source of knowledge with respect to prognostics is data usually presented as cause-specific or relative survival estimates, with their limitations. These probabilities are not able to separate the risk of dying from cancer and the risk of dying from other causes, and the published risk estimates often do not account for tumor characteristics, such as T stage and grade, in addition to age and gender. They are therefore not suitable for clinicians and patients when aiming for risk prognosis. The ideas behind the estimation of crude probabilities of death have been around for more than a decade [Citation8,Citation22] and have been successfully applied to breast and prostate cancer [Citation23,Citation24]. There are ongoing projects to make survival estimates from population-based registries more relevant for clinicians and patients [Citation25].
The use of the loss of life expectancy measure quantifies the individual loss of life expectancy due to a UCB diagnosis. This loss of life expectancy is largest for younger patients and those with the most severe diagnosis. Younger patients have more lifetime that can be lost. Based on the Norwegian data, patients with muscle-invasive bladder cancer diagnosed in their fifties can expect to lose more than a decade of life. The lifetime reduction is much lower for non-muscle-invasive bladder cancers and varies from a few months to 4 years for men and 5 years for women.
There were some challenges when conducting this study. First of all, the grouping into T stages was based only on the first UCB diagnosis of each patient. Thus, all histology and clinical reports within a 4 month window after the first report were taken into consideration. The crude probability of death and the loss of life expectancy can change a lot, for example, in the case of tumor progression. The reported measures need to be understood as average estimates at the time-point of first diagnosis. The variation in the estimators is reflected in the width of the confidence intervals. Another drawback is the incompleteness of the clinical T-stage variable in the data. About one-third of the patients lacked information on T stage at diagnosis, but all patients had information on the morphology, which was used for T-stage classification. Unfortunately, the morphology codes as used by the Cancer Registry of Norway are not able to distinguish between T1 and T2–4 tumors. Complete information was available on age, survival time and gender. Grade information was also available for most cases. All this information was used when imputing the T stage at first diagnosis. To capture the variation in the estimates depending on the imputation, 10 imputed data sets were generated and the presented estimates were derived by pooling the results from all 10 imputed data sets. It is also important to mention that for patients with a UCB diagnosis between 2005 and 2010, 10 year survival estimates are uncertain since observed survival was only available for 4 to less than 10 years. In addition, there was a lack of information on the treatment of the UCB patients, even though treatment has a major impact on survival.
This study presented both the estimates for the crude probability of death and loss of life expectation for specific ages (10%, 50% and 90% percentiles). The underlying framework makes it possible to calculate measures for any other age as well. The analysis could easily be extended for other risk variables, if available, which are considered to be relevant to the survival of the patients.
Besides providing more intuitive measures for individual risk prognosis, the usefulness of these measures was illustrated.
In conclusion, using the suggested framework, these crude probabilities of death can be calculated for any combination of gender, age and T stage at initial diagnosis, and provide thus a more correct and individual prognosis than overall relative survival estimates published by the cancer registries. Furthermore, the expected loss of life expectancy illustrates the consequence for the affected patients and the burden on society. The included probability tables can be used in clinical praxis for risk communication.
Disclosure statement
The authors declare that they have no competing interests.
References
- Cancer Registry of Norway. Cancer in Norway 2014 - Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2015. Available from: https://www.kreftregisteret.no/globalassets/cancer-in-norway/2014/cin2014-special_issue.pdf.
- Socialstyrelsen Cancerfonden. Cancer i siffror 2013 – Populärvetenskapliga fakta om cancer. 2013. Available from: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19108/2013-6-5.pdf.
- Cancer Research UK [Internet]. Bladder cancer statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer (accessed 2014 June 2).
- Engholm G, Ferlay J, Christensen N, Kejs AMT, Johannesen TB, Khan S, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic countries, Version 7.2. Association of the Nordic Cancer Registries. Danish Cancer Society 2016.
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr (accessed 2016 April 15).
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430.
- Gail MH. Estimation and interpretation of models of absolute risk from epidemiologic data, including family-based studies. Lifetime Data Anal 2008;14:18–36.
- Lambert P, Dickman P, Nelson C, Royston P. Estimating the crude probability of death due to cancer and other causes using relative survival models. Stat Med 2010;29:885–95.
- Larsen IK, Smastuen M, Johannesen TB, Småstuen M, Johannesen TB, Langmark F, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31.
- Mostofi F, Sobin L, Tosoni I. Histological typing of urinary bladder tumours. International histological classification of tumours, No 19. Geneva: World Health Organization; 1973.
- Raghunathan TE, Lepkowski JM, Van Hoewyk J, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001;27:85–96.
- Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42.
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987.
- Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91:473–89.
- Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med 2004;23:51–64.
- Nelson CP, Lambert PC, Squire IB, Jones DR. Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med 2007;26:5486–98.
- Rutherford MJ, Crowther MJ, Lambert PC. The use of restricted cubic splines to approximate complex hazard functions in the analysis of time-to-event data: a simulation study. J Stat Comput Simul 2015;85:777–93.
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J 2009;9:265.
- Hakama M, Hakulinen T. Estimating the expectation of life in cancer survival studies with incomplete follow-up information. J Chronic Dis 1977;30:585–97.
- Andersson TML, Dickman PW, Eloranta S, Lambe M, Lambert PC. Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat Med 2013;32:5286–300.
- StataCorp. Stata statistical software: Release 14. College Station, TX: StataCorp; 2015.
- Cronin KA, Feuer EJ. Cumulative cause-specific mortality for cancer patients in the presence of other causes: a crude analogue of relative survival. Stat Med 2000;19:1729–40.
- Lambert PC, Holmberg L, Sandin F, Bray F, Karen M, Linklater KM, et al. Quantifying differences in breast cancer survival between England and Norway. Cancer Epidemiol 2011;35:526–33.
- Eloranta S, Adolfsson J, Lambert PC, Stattin P, Akre O, Andersson TM, Dickman PW. How can we make cancer survival statistics more useful for patients and clinicians: an illustration using localized prostate cancer in Sweden. Cancer Causes Control 2013;24:505–15.
- Feuer EJ, Lee M, Mariotto AB, Cronin KA, Scoppa S, Penson DF, et al. The Cancer Survival Query System: making survival estimates from the surveillance, epidemiology, and end results program more timely and relevant for recently diagnosed patients. Cancer 2012;118:5652–62.