Abstract
Objective: This study aimed to determine the level of misattribution of prostate cancer deaths in Norway based on the county of Vestfold in the years 2009–2014.
Materials and methods: The study included 328 patients registered as dead from prostate cancer (PCD; part I of death certificate), 126 patients with prostate cancer as other significant condition at death (OCD; part II of death certificate) and 310 patients who died with a diagnosis of prostate cancer not registered on the death certificate (PC-DCneg) in Vestfold County in 2009–2014. The complete cohort with patients’ names and dates of birth was provided by the Norwegian Institute of Public Health and the Norwegian Cancer Registry. The true cause of death of all patients was evaluated based on patient journals.
Results: Over-reporting of prostate cancer deaths in the PCD group was 33% while under-reporting in the OCD and PC-DCneg groups was 19% and 5%, respectively. The correlation between registered and observed causes of death was 0.81 (95% confidence interval 0.78–0.83). Misattribution of prostate cancer deaths increased significantly with patient age and decreasing Gleason score.
Conclusions: Prostate cancer mortality statistics in Norway are relatively accurate for patients aged <75 years at death. However, overall accuracy of cause of death assignment is significantly reduced by misattribution among older patients (> 75 years), who represent the large majority of prostate cancer deaths. Over-reporting of prostate cancer deaths among elderly people may not be an exclusively Norwegian phenomenon and may affect prostate cancer mortality statistics in other countries.
Introduction
Official cancer mortality statistics strongly influence the perception of different cancers, their impact on public health and the effects of treatment efforts [Citation1]. The accurate determination of cancer deaths and high quality of death certificates are essential for achieving reliable mortality statistics. However, such high-quality information is commonly only available in clinical trials or hospital series.
Norway and other Scandinavian countries report consistently higher prostate cancer mortality rates than other Western countries [Citation1,Citation2]. Several explanations for this have been suggested, among them a potentially higher underlying risk of prostate cancer death and differences in national health strategies regarding screening and treatment [Citation2]. In 1981, Percy et al. documented that misattribution of cause of death in death certificates may bias mortality statistics [Citation3]. Several studies have since corroborated this evidence [Citation4–6]. For prostate cancer, there is compelling evidence that increasing prevalence due to screening has significantly affected misattribution of prostate cancer deaths [Citation7].
Several Scandinavian studies have addressed the quality of death certificates with regard to prostate cancer, demonstrating relatively reliable results with misattribution rates of 10% or less [Citation8–11]. However, previous audits of prostate cancer deaths have been either registry based [Citation10,Citation11] or conducted in study patients who were considerably younger at death than the majority of men dying of prostate cancer [Citation8,Citation9]. Correct attribution of the underlying cause of death becomes more challenging with increasing age owing to competing comorbidities [Citation12]. A recent Norwegian study, comparing relative and cause-specific survival for several cancer sites, documented significant differences for older prostate cancer patients, suggesting incorrect coding of the underlying cause of death [Citation13].
Increasing prostate cancer incidence rates in Norway and the likely corresponding increase in prevalence of non-lethal cancers may potentially exacerbate this problem of misattribution bias.
The aim of this population-based study was to determine the level of misattribution of Norwegian prostate cancer deaths based on data from the county of Vestfold in the years 2009–2014.
Materials and methods
Study population
Vestfold Hospital Trust is the only hospital in Vestfold County, which has approximately 230,000 inhabitants representing approximately 5% of the Norwegian population. Vestfold County has a somewhat higher proportion of men older than 70 years than the rest of Norway, but is otherwise representative of the entire country in terms of relevant socioeconomic factors (see supplementary material).
For the 6 year period 2009–2014, names and dates of birth of all deceased men in Vestfold County, registered with either prostate cancer as the immediate/underlying cause of death (part I of the death certificate, n = 341) or another significant condition at death (part II of the death certificate, n = 127), were obtained from the Norwegian Institute of Public Health, while data on all deceased men with prostate cancer whose diagnosis was not mentioned on the death certificate were obtained from the Norwegian Cancer Registry.
Prostate cancer care for almost all patients in the county of Vestfold is provided at Vestfold Hospital Trust, including diagnosis, treatment and follow-up. Patients from two peripheral municipalities, who routinely receive their prostate cancer care elsewhere, were excluded from the study. This resulted in a study population of 328 patients with prostate cancer as an immediate or underlying cause of death (part I of the death certificate), referred to as PCD; 126 patients with prostate cancer as other significant condition (part II of the death certificate), referred to as OCD; and 310 patients with prostate cancer without any mention of prostate cancer on the death certificate, referred to as PC-DCneg (Norwegian Cancer Registry).
Assessment of cause of death
A review committee was formed consisting of three urologists and one oncologist, all of whom were experienced in treating prostate cancer patients. Three consecutive reviews of the study population were conducted. Patient hospital records provided sufficient information on medical histories for the great majority of patients. For 12 patients, additional information had to be obtained from nursing homes or family doctors.
First review of patient history
The first review served as a filtering process and identified patients with an unambiguous, immediate cause of death and a clear underlying disease process, separating out patients ascribed as either dead from prostate cancer (PCD) or dead from other causes (OCD). The review was conducted by the first author, with a blinded audit of 50 random patients in the sample by two other committee members demonstrating perfect correlation.
Prostate cancer death was assumed when the immediate cause of death was caused by systemic or local complications of the disease process or cancer-directed treatment and comorbidities were either absent or only of minor importance. Death from other causes was defined by the criteria listed in .
Table 1. First review of patient history: definition of death from other causes for patients with prostate cancer as underlying of immediate cause of death (PCD, n = 328) and corresponding results.
Patients who could not be placed with certainty in either of the above categories were included in the second review for further evaluation.
Patients who had been autopsied were registered with the underlying cause of death given on the autopsy report, and no further review of the cause of death was conducted (n = 16).
Second review of patient history
Patients were independently reviewed by committee members unaware of the other reviews. Prostate cancer death was assumed when the immediate cause of death was due to systemic or local complications of the disease process or cancer-directed treatment. The following categories were assigned, answering the question of whether the patient’s death was due to prostate cancer: ‘Yes/No’ (preferable category), ‘Likely/Unlikely’ (if a definite answer was not possible) or ‘Not possible to determine’ (if any qualified answer was impossible to give, owing to numerous and competing comorbidities). Patients on whom committee members reached conflicting conclusions were included in the third review.
Third review of patient history
Patients whose underlying cause of death was still undetermined after the first two reviews were discussed in a consensus meeting with all members of the committee present. Patient histories were reviewed in plenum and a consensus decision was reached upon which all committee members could agree. Assignment of labels followed the same rules as outlined under the second review of patient history.
Final result
All patients with the label ‘Yes’ or ‘Likely’ after the third review were collapsed into the final Yes category (dead from prostate cancer). Patients labeled ‘No’ or ‘Unlikely’ were collapsed into the final No category (not dead from prostate cancer). ‘Not possible to determine’ was upheld as an independent category.
Statistics
Baseline data and prostate cancer death misclassification rates were described by median, range and percentages. Confidence intervals (CIs) for percentages were calculated using Wilson score interval with Yates’ continuity correction. The effect of age and study year on the risk of misclassifying cause of death was studied by binary logistic regression. Subgroup analysis was performed by dividing patients into seven age groups (< 65, 65–70, 70–75, 75–80, 80–85, 85–90, and >90 years). All statistical analyses were conducted using SPSS statistics, version 23 (IBM Corp., Armonk, NY, USA) and the R statistical package [Citation14]. Correlation between the cause-of-death methods was calculated using Cohen’s kappa.
Ethics
The study was approved by the Regional Committee for Medical Research Ethics (REK).
Results
The median age at death was 83–84 years in all groups. In the PCD group, 21% of patients were younger than 75 years at death, with corresponding numbers of 14% in the OCD group and 16% in the PC-DCneg group (n = 70, n = 18, n = 50), while 14% of patients in the PCD group, 20% in the OCD group and 14% in the PC-DCneg group were older than 90 years at death (n = 46, n = 25, n = 43). Dementia had been diagnosed in 18% of the PCD patients, 11% of the OCD patients and 10% of the PC-DCneg patients (n = 58, n = 14, n = 32). Fifteen patients in both the PCD and OCD groups and two patients in the PC-DCneg group had no clinical or histological diagnosis of prostate cancer. The clinical characteristics of all patients are listed in .
Table 2. Comparison of clinical characteristics of patients with prostate cancer as the immediate or underlying cause of death according to the death certificate (PCD), patients with prostate cancer as other significant condition at death (OCD) and patients with prostate cancer with no mention of the diagnosis on the death certificate (PC-DCneg).
Death from prostate cancer (PCD)
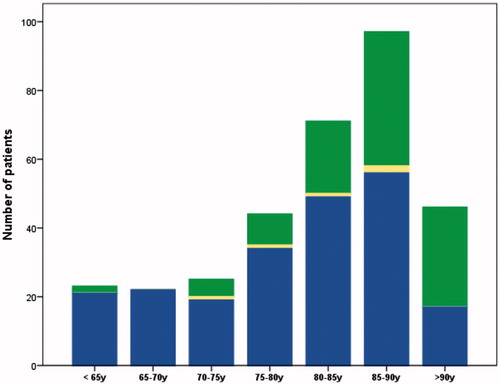
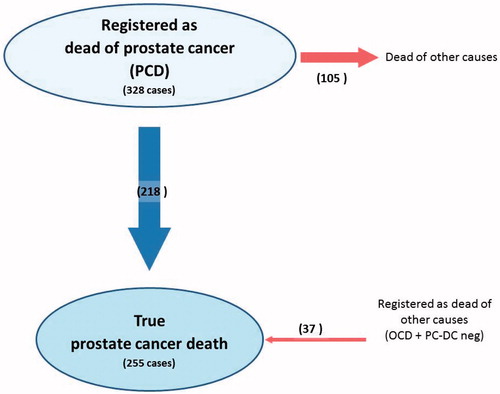
The first review of the PCD group identified 21% of patients (n = 70) who had died of causes other than prostate cancer. Their prostate cancer status at death is listed in . The final result of the review process demonstrated that 32% of patients (n = 105) in the PCD group had died of other causes. Logistic regression demonstrated a statistically significant increase in misattribution rates with increasing age in the PCD group (p < 0.001, OR 1.8 per 5 years, 95% CI 1.4–2.1). Among patients younger than 75 years, 10% (7/70) were incorrectly labeled as dead from prostate cancer while the misattribution rate increased to 63% in patients aged 90 years and older (29/46). Misattribution of cause of death per age group in the PCD group is illustrated in . Logistic regression revealed a statistically significant impact on misattribution rates by Gleason grade (p = 0.007, OR 0.7, 95% CI 0.5–0.9). There was no statistically significant variation in misattribution rates for the years of diagnosis 2009–2014 (p = 0.25, OR 1.1, 95% CI 1.0–1.2) or for tumor stage at diagnosis (p = 0.12, OR 0.8, 95% CI 0.6–1.1).
Death from other causes (OCD)
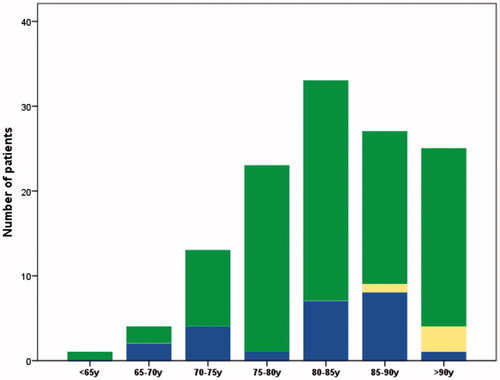
In the OCD group, the final results of the review process indicated that 18% of patients (n = 23) had died of prostate cancer. Logistic regression showed a statistically non-significant decreasing trend of misattribution by age in the OCD group (p = 0.33, OR 1.2 per 5 years, 95% CI 0.9–1.6). Misattribution of cause of death per age group in the OCD group is illustrated in . Gleason grade had a statistically significant impact on misattribution (p = 0.006, OR 0.5, 95% CI 0.3–0.8). There was no statistically significant variation in misattribution rates for year of diagnosis (p = 0.18, OR 0.8, 95% CI 0.6–1.1) or stage at diagnosis (p = 0.25, OR 0.7, 95% CI 0.4–1.3).
Figure 2. Total number of patients registered as dead from other causes per age group: patients dead from prostate cancer (blue), patients dead from other causes (green) and patients whose cause of death was not possible to determine (yellow).
The review process of PCD and OCD patients and its results are illustrated in and .
Table 3. Results of the review process for patients with prostate cancer as the immediate or underlying cause of death (PCD) (n = 328).
Table 4. Results of the review process for patients with prostate cancer as other significant condition at death (OCD) (n = 126).
Patients with prostate cancer not registered on the death certificate (PC-DCneg)
Among the 310 PC-DCneg patients, the review process identified 5% (n = 14) who had died of prostate cancer.
Underreporting of prostate cancer deaths in the OCD and PC-DCneg groups was 18% and 5%, respectively, while over-reporting in the PCD group was 32% (). The correlation between reported patient death and observed patient death was 0.81 (95% CI 0.78–0.83), with Cohen’s kappa (0.4, 95% CI 0.3–0.5) showing a moderate correlation between registered and true prostate cancer death.
Discussion
In a cohort of 328 consecutive patients with prostate cancer as cause of death, this study found that one-third died of other causes, while among 436 patients with prostate cancer who died of other causes approximately one-tenth died of prostate cancer. The net result was a considerable over-registration of prostate cancer deaths in Vestfold County for the years 2009–2014.
This study has several strengths: its population-based design, the consecutive cohort of patients, the 6 year study period and rich clinical data for the majority of patients. The weaknesses of the study are its limited geographical scope and the difficult clinical decision-making process, which might be biased. The committee members in this study treat prostate cancer patients daily. A panel of physicians from other specialties may have come to different conclusions for some of the study patients.
The findings seem to contradict previous studies which documented relatively reliable mortality numbers for prostate cancer in Norway [Citation10,Citation11]. However, these studies were register based and evaluated prostate cancer deaths that occurred in 1996, before the PSA-induced increase in prostate cancer prevalence. Feuer et al. demonstrated during the early PSA era that rising prostate cancer prevalence rates were mirrored directly by corresponding changes in mortality rates [Citation7]. The authors hypothesized that a fixed percentage of the rising and falling pool of newly diagnosed prostate cancer patients was mislabeled as dying of the disease [Citation7]. According to this theory, it is likely that misattribution rates in Norway have increased considerably during the past decade (2004–2013) as prostate cancer prevalence has doubled at least partly owing to extensive PSA testing [Citation15]. Prostate cancer prevalence is equal in Vestfold County and on the national level, suggesting that misattribution rates may be comparable [Citation16]. A more recent study by the Norwegian Cancer Registry demonstrated that relative survival estimates for prostate cancer were consistently above cause-specific survival estimates for all but the very youngest patients, with the most marked differences among the oldest patients (>85 years) [Citation13]. One explanation suggested by the authors is that prostate cancer patients in Norway are somewhat healthier than the general population. However, as the observed effect is strongest among the oldest patients, the reported differences are more likely to be due to incorrect coding of the underlying cause of death. This is mirrored by the patients in the present study whose cause of death could not be determined during the first review: the median age in this subgroup was 86 years and for the majority a cause of death could first be determined after the third review. For patients younger than 75 years, on the other hand, results were relatively reliable, with a 90% concordance between cause of death on the death certificate and cause of death based on committee evaluation. However, these patients were a clear minority in the PCD group (21%), where 75% of patients were older than 77 years at death.
A further explanation for the high misattribution rates in this study may be found in the Norwegian proceedings for death certification [Citation17,Citation18]. In contrast to many other Western countries, death certificates in Norway are filled out by the doctor who confirms the patient’s death. These are usually the most junior doctors at the hospitals or on-call doctors at the municipal primary care emergency departments and nursing homes, who rarely have intimate knowledge of the patient’s medical history. In 1986, 57% of Norwegian prostate cancer deaths occurred in hospitals, where determination of the cause of death is usually more accurate [Citation19], while only 29% occurred in nursing homes. By 2014 these numbers were reversed, with more than 60% of prostate cancer deaths occurring in nursing homes and only 23% in hospitals (2016 mail correspondence between the Norwegian Institute of Public Health and the first author). This change may have impacted the accuracy of death certificates considerably. Furthermore, it seems that not all doctors are fully confident with the concepts of immediate, underlying and contributory causes of death. In addition, the guidelines from the WHO state that in certain circumstances a specific diagnosis in part II of the death certificate may be registered as the underlying cause of death instead of an unspecific diagnosis in part I. This will have little impact on the actual cause of death in patients with a cancer diagnosis and few or no comorbidities. However, in patients with several comorbidities, usually older patients, this lack of knowledge may have a considerable impact on accuracy.
A key issue is the interpretation of the results and the question of whether over-registration of prostate cancer deaths is a specific Norwegian problem. In this case, prostate cancer mortality may not be significantly higher in Norway than in other Western countries. A Swedish registry-based study, evaluating the quality of official cause of death diagnoses of prostate cancer patients diagnosed in 1987–1999 and deceased before 2003, documented a relatively high reliability (correlation of 0.86%) of official cause of death statistics [Citation20]. However, the hallmarks of misattribution of cause of death are similar to those in the present study, with high correlation among the youngest patients and increasing misattribution with increasing age. The higher misattribution rates seen in the current study may be due to the more recent timeframe of this study, with higher underlying prevalence rates of prostate cancer.
Further results of death certificate audits have been published for Finnish, Swedish and UK prostate cancer patients [Citation8,Citation9,Citation21,Citation22], demonstrating high accuracy of death certificates in those countries, while a similar evaluation in a group of US prostate cancer patients showed more ambivalent results [Citation23]. However, patients evaluated in these studies were participants in clinical trials and were on average significantly younger at death than prostate cancer patients in the general population (maximum age at death 74–77 years in the Finnish and Swedish clinical trials). The majority of prostate cancer deaths occur in patients older than 80 years and thus similar quality issues as described in the present study may negatively affect the prostate cancer mortality statistics in the above-mentioned countries. In this case, prostate cancer mortality may still be relatively high in Norway, with excess mortality among patients older than 80 years. This may be due to national therapy recommendations where, until recently, a strict age limit (75 years) for recommending radical therapy has been observed.
Prostate cancer mortality statistics in Norway must be interpreted with caution and may have to be supplemented by additional parameters (e.g. statistics on patients with castration-resistant prostate cancer) that may better reflect disease burden and intervention effects in the general population.
Sven_L_ffeler_et_al_supplemental_content.zip
Download Zip (33.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27.
- Kvale R, Auvinen A, Adami HO, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99:1881–1887.
- Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71:242–250.
- Alfsen GC, Lyckander LG, Lindboe AW, et al. [Quality control of deaths in hospitals]. Tidsskr nor Laegeforen. 2010;130:476–479.
- Johansson LA, Westerling R. Comparing hospital discharge records with death certificates: can the differences be explained?. J Epidemiol Community Health. 2002;56:301–308.
- Maudsley G, Williams EM. 'Inaccuracy' in death certification-where are we now? J Public Health Med. 1996;18:59–66.
- Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer-part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst. 1999;91:1025–1032.
- Godtman R, Holmberg E, Stranne J, et al. High accuracy of Swedish death certificates in men participating in screening for prostate cancer: a comparative study of official death certificates with a cause of death committee using a standardized algorithm. Scand J Urol Nephrol. 2011;45:226–232.
- Makinen T, Karhunen P, Aro J, et al. Assessment of causes of death in a prostate cancer screening trial. Int J Cancer. 2008;122:413–417.
- Hernes E, Harvei S, Glattre E, et al. High prostate cancer mortality in Norway: influence of Cancer Registry information? APMIS. 2005;113:542–549.
- Hernes E, Johansson LA, Fossa SD, et al. High prostate cancer mortality in Norway evaluated by automated classification of medical entities. Eur J Cancer Prev. 2008;17:331–335.
- Havlik RJ, Rosenberg HM. The quality and application of death records of older patients. New York: Oxford University Press; 1992.
- Skyrud KD, Bray F, Moller B. A comparison of relative and cause-specific survival by cancer site, age and time since diagnosis. Int J Cancer. 2014;135:196–203.
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016.
- Cancer Registry of Norway. Årsrapport 2004-2013 [Norwegian]. 2014. Available from: https://www.kreftregisteret.no/globalassets/publikasjoner-og-rapporter/arsrapporter/2015/aarsrapport_2015_prostata.pdf
- Larsen IK, Myklebust TA, Robsahm TE, et al. Spesialnummer: Kreft i Norges fylker 1954–2013. I Cancer in Norway 2013-Cancer incidence, mortality, survival and prevalence in Norway. 2015. p. 181.
- Bakken IJ, Ellingsen CL, Pedersen AG, et al. Comparison of data from the Cause of Death Registry and the Norwegian Patient Register. Tidsskr nor Laegeforen. 2015;135:1949–1953.
- Pedersen AG, Ellingsen CL. Data quality in the Causes of Death Registry. Tidsskr nor Laegeforen. 2015;135:768–770.
- Albertsen PC, Walters S, Hanley JA. A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol. 2000;163:519–523.
- Fall K, Stromberg F, Rosell J, et al. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42:352–357.
- Maattanen L, Auvinen A, Stenman UH, et al. European randomized study of prostate cancer screening: first-year results of the Finnish trial. Br J Cancer. 1999;79:1210–1214.
- Turner EL, Metcalfe C, Donovan JL, et al. Contemporary accuracy of death certificates for coding prostate cancer as a cause of death: is reliance on death certification good enough? A comparison with blinded review by an independent cause of death evaluation committee. Br J Cancer. 2016;115:90–94.
- Barry MJ, Andriole GL, Culkin DJ, et al. Ascertaining cause of death among men in the prostate cancer intervention versus observation trial. Clinical Trials. 2013;10:907–914.