Abstract
Objectives: The aim of this study was to examine the use of abiraterone and enzalutamide, two oral novel antiandrogens (NOVAs), in men with prostate cancer (PCa) in Sweden.
Materials and methods: This cross-sectional study investigated filled prescriptions for NOVAs recorded in the Swedish Prescribed Drug Register between July 2015 and April 2016. Associations between age, comorbidity, educational level, marital status and county of residence and filled prescriptions were analyzed in the National Prostate Cancer Register (NPCR) and other health population-based registers, using multivariable logistic regression.
Results: Of 91,209 men, 1650 (2%) had at least one prescription filled for NOVAs, of whom 1350 (82%) had high-risk or metastatic PCa at diagnosis.. Of 1914 men with M1 disease and a high probability of castration-resistant prostate cancer (CRPC), 22% had a prescription for NOVAs at a median 3 years after the date of diagnosis. At multivariable logistic regression analysis,, the likelihood of NOVA use was lower in older men [age >80 vs <70 years: odds ratio (OR) 0.23, 95% confidence interval (CI) 0.15–0.35] and in men with lower educational level (high vs low education: OR 1.64, 95% CI 1.23–2.20). There was up to a five-fold difference in the use of NOVAs between county councils.
Conclusions: Less than one-third of potentially eligible men with CRPC received NOVAs in 2015–2016. There were large differences in use according to age and region of residence, indicating that efforts are needed to improve equal access to novel cancer drugs.
Introduction
Several drugs have been shown to increase survival and improve the quality of life in men with castration-resistant prostate cancer (CRPC) [Citation1–5]. These drugs include taxane-based chemotherapy, radiopharmaceuticals, and enzalutamide and abiraterone acetate, two novel antiandrogens (NOVAs). The effectiveness of NOVAs has been documented both before and after docetaxel treatment in symptomatic and asymptomatic men, regardless of age. However, the uptake of NOVAs has been slow in many countries owing to the high costs and the large and increasing number of men with CRPC who are potentially eligible for treatment with NOVAs. The duration of time spent in the CRPC state has increased in recent years, with a current median estimate of 2 years [Citation3,Citation4,Citation6]. Based on the fact that more than 95% of men who die of prostate cancer (PCa) in Sweden have been treated with androgen deprivation therapy (ADT) before death [Citation7], it can be inferred that virtually all men who die of PCa have been in the CRPC state before death. Assuming a median survival in the CRPC state of 2 years, 2500 PCa deaths per year, linear trends in survival and that all men in the CRPC state die of PCa, a crude estimate of the number of men in Sweden in the CRPC state would be approximately 5000. While large variations in the use of other costly cancer treatments have been reported [Citation8], little is known about NOVA use on a population level.
This study examined the use of NOVAs by investigating information on filled (dispensed) prescriptions in the Prescribed Drug Register and other nationwide population-based healthcare registers and demographic databases in Sweden. More specifically, the aim of the study was to assess approximately: (i) the proportion of men with CRPC who receive NOVAs; (ii) the PCa trajectory before NOVA use; and (iii) factors associated with the receipt of NOVAs in men with CRPC in a real-world setting.
Materials and methods
Data collection
The National Prostate Cancer Register (NPCR) of Sweden has captured 98% of all incident PCa cases in Sweden since 1998, compared to the Cancer Register, to which registration is mandated by law. The NPCR contains detailed information on PCa characteristics and primary treatment but holds no information on subsequent disease trajectory, so men with CRPC cannot be identified in the NPCR [Citation9]. In this study, risk categories of PCa were clustered into favorable-risk and aggressive disease ().
Table 1. Characteristics of men in Prostate Cancer data Base Sweden (PCBaSe) RAPID with prostate cancer registered between 1 January 1998 and 31 December 2014, in the National Prostate Cancer Register and residing in Sweden on 1 July 2015.
Using the individually unique personal identity numbers assigned to all residents of Sweden, data in the NPCR were linked to information in the Prescribed Drug Register, the Patient Register, the Cause of Death Register, the Longitudinal database on socioeconomic factors (LISA, acronym in Swedish) and the Register of Total Population and Population changes in the Prostate Cancer data Base Sweden (PCBaSe) RAPID in May 2016.
Information on filled prescriptions was retrieved from the Prescribed Drug Register, which is updated monthly and captures virtually all filled prescriptions in Sweden, with data on patient identity missing for less than 0.3% of the prescriptions [Citation9]. Dispensation of drugs in hospital or nursing homes settings is not recorded in the Prescribed Drug Register. Filled prescriptions for abiraterone (ATC code L02BX03) and enzalutamide (L02BB04) have been recorded since July 2015.
The burden of concomitant disease was assessed based on the Charlson Comorbidity Index (CCI), estimated using data on discharge diagnoses available in the Patient Register up to 1 July 2015, as described previously [Citation9,Citation10].
Information on educational level and income was retrieved from the LISA database. Men were categorized according to the highest attained level of education, into low (< 10 years of education), intermediate (10–12 years) and high (> 12 years), and income was assessed in quartiles.
In Sweden, healthcare is funded by taxation and provided by 20 independent county councils. There is a national process for the introduction of new drug therapies in Sweden in which all county councils, several governmental agencies and the pharmaceutical industry collaborate. The Dental and Pharmaceuticals Benefits Agency (TLV) is responsible for the health economic evaluation. A pharmaceutical company applies for reimbursement for a drug to the TLV and the Pharmaceutical Benefits board decides whether the drug should be subsidized. The New Therapies (NT) council makes recommendations and formulates follow-up protocols for the use of new drug therapies after market authorization. However, the decisions of the NT council and the national guidelines are merely recommendations, and the county council can decide whether or not to follow them.
Design and statistical methods
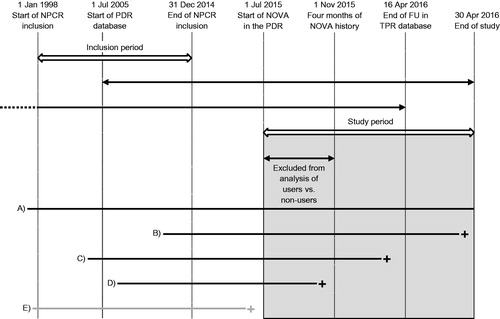
This was a cross-sectional study on prescriptions for NOVAs that were filled between 1 July 2015 and 30 April 2016 by men who had been diagnosed with PCa in 1998–2014 and registered in the NPCR, and who resided in Sweden on 1 July 2015 (). The maximum number of doses in a prescription in Sweden covers 3 months of drug consumption. Therefore, a 4 month run-in period was used to ensure that men did not use NOVAs from prescriptions filled in an earlier calendar period. Men who died or were censored before 1 November 2015 were excluded from comparisons of NOVA users and non-users, since the follow-up was too short. The filling of prescriptions for NOVA was analyzed using logistic regression in univariable and multivariable models including calendar year of diagnosis, age at diagnosis, comorbidity, educational level, marital status and the presence of a university hospital in the county of diagnosis, and presented as odds ratios (ORs) with 95% confidence intervals (CIs).
Figure 1. Timeline of the study period in the Prostate Cancer data Base Sweden (PCBaSe) RAPID. NPCR: National Prostate Cancer Register; PDR: Prescribed Drug Register; NOVA: novel antiandrogen; FU: follow-up; TPR: Total Population Register (vital status). The grey rectangle represents the study period for filled prescriptions for NOVAs in the PDR. A–E illustrate the different periods during which a patient could be at risk before death or censoring: patient A was at risk during the whole study period; patients B and C died during the study period; patient D died before 1 November 2015 and is not included in the analysis of users vs non-users; patient E died before 1 July 2015 and is not included in the study.
Results
Of 91,209 men registered in the NPCR between 1998 and 2014 who resided in Sweden on 1 July 2015, 1650 men (2%) had a record of having at least one prescription for NOVA filled between 1 July 2015 and 30 April 2016 (). Specifically, 518 out of 1650 men (31%) had a prescription for abiraterone, 1210 (73%) for enzalutamide and 78 men (5%) had prescriptions for both drugs.
The median time from the date of PCa diagnosis to the first NOVA prescription during the study period was 6 years [interquartile range (IQR) 3.3–10.3], and 75% of men were above the age of 70 years at the date of their first filled prescription. There were minor differences between men who received NOVAs and all men with PCa regarding comorbidity, marital status, educational level and income at time of diagnosis. Among men who had received NOVAs, 1350 (82%) had aggressive PCa at the date of diagnosis, including the risk categories high-risk, locally advanced, or regional or bone metastases, and 1019 (68%) had received ADT as primary treatment. In comparison, only 13,593 (15%) of all men with PCa had received primary ADT.
To investigate factors associated with the receipt of NOVAs, 1914 men with a high probability of CRPC were identified, defined by distant metastases at the date of diagnosis, receipt of gonadotropin-releasing hormone (GnRH) agonists on 31 December 2014 and risk on 1 November 2015 (. Of these men, 421 (22%) had had a prescription for a NOVA filled at a median time of 3 years (IQR 2–5 years) after diagnosis; 274 of these 421 men (64%) were aged 70 years or older when they had their first prescription for a NOVA filled. There was a strong decrease in the likelihood of receiving NOVAs with increasing age (age >80 vs <70 years: odds ratio (OR) 0.23, 95% confidence interval (CI) 0.15–0.35, ). The highest proportion of men receiving NOVA was observed in men below the age of 70 years with no comorbidities at diagnosis, among whom 106 out of 369 (29%) received NOVAs; the proportion was similar for men aged 70–79 years with no comorbidities, 114 of 476 men (24%), whereas among men above the age of 80 with no comorbidities, only 37 out of 267 men (14%) received NOVAs (Supplementary Figure 1). There was no association between the CCI and use of NOVAs except for in men above 80 years, of whom only 7% of men with CCI =3 or higher received NOVAs (Supplementary Figure 1).
Table 2. Characteristics of men with a high probability of castration-resistant prostate cancer in the Prostate Cancer data Base Sweden (PCBaSe) RAPID, registered in the National Prostate Cancer Register between 1 January 1998 and 31 December 2014 with distant metastases at the date of diagnosis, on gonadotropin-releasing hormone (GnRH) therapy on 31 December 2014 and residing in Sweden on 1 November 2015.
Table 3. Odds ratios (ORs) and 95% confidence intervals (CIs) for receipt of a novel antiandrogen (NOVA).
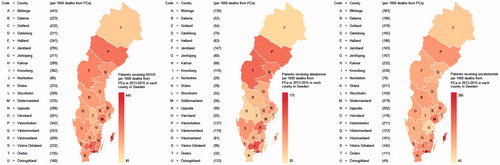
In a subsequent step, the number of men who had had prescriptions for NOVA filled was estimated per 1000 PCa deaths (). This denominator was used to obtain an approximate measure of the expected number of men with CRPC [Citation11]. There was a five-fold difference in the use of NOVAs between the county with the lowest use of NOVAs (86 per 1000 PCa deaths) and that with the highest use (443 per 1000 PCa deaths). In three of the most populous counties, the use of NOVAs was quite similar: Skåne (233 per 1000 PCa deaths), Västra Götaland (232 per 1000 PCa deaths) and Stockholm (209 per 1000 PCa deaths). There was also evidence of large differences between counties with regard to primary choice of abiraterone or enzalutamide.
Figure 2. Proportion of men with prostate cancer (PCa) diagnosed in 2014 who had a prescription filled for (a) a novel antiandrogen (NOVA), (b) abiraterone or (c) enzalutamide, between 1 July 2015 and 30 April 2016 per 1000 deaths from PCa in each county in 2013–2015.
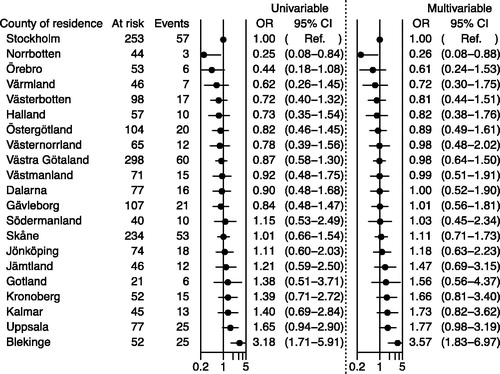
shows the likelihood of NOVA use in multivariable analysis according to county of residence at diagnosis, with Stockholm county council as reference. The ORs varied from 0.26 (95% CI 0.08–0.88) for the county with the lowest probability of NOVA use to 3.57 (95% CI 1.83–6.97) for the county with the highest use, paralleling the crude estimates reported in .
Figure 3. Odds ratios (ORs) and 95% confidence intervals (CIs) for receipt of novel antiandrogens (NOVAs) according to county of residence: logistic regression model including men registered in the National Prostate Cancer Register (NPCR) between 1 January 1998 and 31 December 2014 with metastatic cancer, on gonadotropin-releasing hormone (GnRH) agonist on 31 December 2014. All men included in the figure were at risk on 1 November 2015. Filling of a prescription for a novel antiandrogen (NOVA), abiraterone or enzalutamide, was determined between 1 July 2015 and 15 April 2016. A logarithmic scale (base 10) was used. The adjusted model includes year of diagnosis, age, comorbidity, marital status and educational level.
During the study period, the use of enzalutamide increased three-fold whereas the use of abiraterone remained constant (Supplementary Figure 2).
Discussion
The results of this nationwide population-based study in Sweden on filled prescriptions for abiraterone and enzalutamide, two NOVAs, suggest that less than one-third of men with CRPC used NOVAs at any time-point in 2015–2016, with a wide range in use, most strongly related to age and healthcare provider. There was a five-fold difference in NOVA use according to age and county of residence.
Limitations of this study include that the proportion of men with CRPC who had a NOVA prescription filled could only be indirectly estimated, since no data on the prevalence of CRPC in Sweden are available and survival times for patients with CRPC in contemporary clinical practice are unknown. However, the estimate based on filled NOVA prescriptions in men with a high probability of CRPC was quite close to an estimate based on an assessment of around 5000 men in Sweden living with CRPC which, combined with the number of filled prescriptions during the study period, would translate into an approximately 30% uptake of NOVAs in Sweden. While these estimates are based on a number of assumptions that are not entirely true, they are still useful in that they provide for the first time a rough estimate of the use of NOVAs in men with CRPC in a large population-based cohort. Taken together, these estimates suggest that less than one-third of men who are potentially eligible for NOVAs had at least one prescription filled for this type of drug. No data were available on use of chemotherapy for PCa as this therapy is delivered in hospital and not included in the Prescribed Drug Register; thus, NOVA use could not be divided into pre- and post-chemotherapy. Drug survival could not be estimated since the study period was too short. Furthermore, it was not possible to assess NOVA receipt according to performance status; however, CCI was used to assess general health status in relation to NOVA receipt. Strengths of this study include the use of a nationwide population-based cohort with comprehensive data from several high-quality healthcare registers and demographic databases, with the virtually complete capture of all diagnoses and treatments during a recent calendar period [Citation9,Citation12,Citation13].
The data showed that most men who had prescriptions for NOVAs filled had advanced PCa at diagnosis, a majority had received ADT as primary PCa treatment, and a majority of men were above the age of 70 years when they received their first NOVA prescription. Men on NOVAs in this study were slightly younger than men in the COU-AA-301 and COU-AA-302 studies on abiraterone (median age: 68, 69 and 70 years, respectively) [Citation14,Citation15]. In the present study, 82% of men on NOVAs had aggressive disease at diagnosis, whereas only 51% of men in the COU-AA-301 study had a Gleason score ≥8, with similar differences in comparison to the AFFIRM and PREVAIL studies on enzalutamide [Citation5].
Two factors were strongly related to NOVA use: age and county of residence. Despite healthy old men experiencing treatment outcomes similar to those in younger men after chemotherapy and NOVAs [Citation16–19], and older men being equally motivated to receive chemotherapy for a survival benefit, older age in this study was associated with much lower NOVA use [Citation20]. The present group has previously reported less use of chemotherapy in older men with CRPC [Citation11]: 61% of men with CRPC younger than 70 years received chemotherapy, compared to only 5% of men above 79 years of age. These findings are in line with other studies on PCa, as well as on breast and colorectal cancer [Citation21–23], in which older patients were less likely than younger patients to receive aggressive treatment. In contrast, high comorbidity did not decrease NOVA use, except in men above 80 years of age. The present data indicate that decisions on the use of NOVAs were mostly based on chronological age; however, life expectancy based on biological age, i.e. age and comorbidity, should guide treatment selection in men with advanced PCa [Citation24].
In addition, the use of NOVA prescriptions was higher in men with a high educational level than in men with low education, corroborating earlier findings of more intense work-up and active treatment associated with an high educational level [Citation25–27]. Educational level is likely to be related not only to health awareness and healthcare-seeking behavior, but also to patient–clinician interactions [Citation25].
This investigation found more than a five-fold difference between counties in the filling of NOVA prescriptions, differences that could not be explained by, for example, the presence of or proximity to a university hospital. To facilitate uptake, the national guidelines state that patients who are potentially eligible for NOVAs should be discussed at a multidisciplinary team conference with urologists and oncologists present. Unfortunately, the availability of these multidisciplinary team conferences for men with advanced PCa in different counties in Sweden is unknown.
National recommendations on the use of NOVAs have been in place in Sweden since June 2015, but the present findings indicate a very wide variation between county councils in their adherence to recommendations.
These results are in line with a recent report of striking inequalities in access to oncological drugs in the non-curative setting in many countries across Europe for different types of cancer, including PCa [Citation8].
To the best of the authors’ knowledge, no other nationwide population-based cohort has provided similar information on the use of NOVAs in a real-world setting. Previously, a US study based on electronic medical records in general practice on 809 men who initiated NOVA treatment between 2012 and 2014 reported that abiraterone (74%) was more commonly prescribed than enzalutamide (26%); this could be explained by the earlier approval date for abiraterone [Citation28]. While there were large regional variations in the present study, more prescriptions for enzalutamide than abiraterone were observed, despite there being no recommendation in favor of enzalutamide over abiraterone. The higher and increasing use of enzalutamide is likely to be related to convenience. Potassium levels need to be monitored in men on abiraterone, prednisolone needs to be used concomitantly and there are more food restrictions with abiraterone than for enzalutamide, and these factors may discourage the use of abiraterone for practical reasons not related to oncological effectiveness.
In conclusion, less than one-third of men with PCa in Sweden who were potentially eligible for NOVAs had a prescription for a NOVA filled in 2015–2016. There were large differences in the use of NOVAs according to age and healthcare provider, and to a lesser extent educational level. Efforts are needed to improve equal access to novel cancer drugs in Sweden, including stronger adherence to national guidelines and recommendations for treatment of PCa.
Ingela_Franck_Lissbrant_et_al_supplemental_content.zip
Download Zip (176.7 KB)Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, David Robinson, Mats Andén, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, Ola Bratt, René Blom, Lars Egevad, Calle Waller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin and Karin Hellström.
Disclosure statement
The authors have nothing to disclose.
Additional information
Funding
References
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
- Scher HI, Solo K, Valant J, et al. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One. 2015;10:112.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197.
- James ND, Spears MR, Clarke NW, et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur Urol. 2015;67:1028–1038.
- The National Board of Health and Welfare - Socialstyrelsen. Cause of Death n.d [cited 2016 September 26]. Available from: http://www.socialstyrelsen.se/statistics/statisticaldatabase/causeofdeath.
- Cherny N, Sullivan R, Torode J, et al. ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol. 2016;27:1423–1443.
- Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42:956–967.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Lissbrant IF, Garmo H, Widmark A, et al. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013;52:1593–1601.
- Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol. 2015;54:158–163.
- Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. 2015;51:101–111.
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160.
- Italiano A, Ortholan C, Oudard S, et al. Docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-resistant prostate cancer. Eur Urol. 2009;55:1368–1375.
- Smith MR, Rathkopf DE, Mulders PFA, et al. Efficacy and safety of abiraterone acetate in elderly (75 years or older) chemotherapy naive patients with metastatic castration resistant prostate cancer. J Urol. 2015;194:1277–1284.
- Graff JN, Baciarello G, Armstrong AJ, et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naive metastatic castration-resistant prostate cancer: results from PREVAIL. Ann Oncol. 2016;27:286–294.
- Droz J-P, Aapro M, Balducci L, et al. Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol. 2014;15:e404–e414.
- Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J Natl Cancer Inst. 1994;86:1766–1770.
- Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. JCO. 2010;28:2038–2045.
- Dotan E, Browner I, Hurria A, et al. Challenges in the management of older patients with colon cancer. J Natl Compr Canc Netw. 2012;10:213–224.
- Anderson J, Van Poppel H, Bellmunt J, et al. Chemotherapy for older patients with prostate cancer. BJU Int. 2007;99:269–273.
- Jacobs BL, Lopa SH, Yabes JG, et al. Association of functional status and treatment choice among older men with prostate cancer in the Medicare Advantage population. Cancer. 2016;122:3199–3206.
- Berglund A, Garmo H, Robinson D, et al. Differences according to socioeconomic status in the management and mortality in men with high risk prostate cancer. Eur J Cancer. 2012;48:75–84.
- Krupski TL, Kwan L, Afifi AA, et al. Geographic and socioeconomic variation in the treatment of prostate cancer. JCO. 2005;23:7881–7888.
- Eaker S, Halmin M, Bellocco R, et al. Social differences in breast cancer survival in relation to patient management within a National Health Care System (Sweden). Int J Cancer. 2009;124:180–187.
- Malangone-Monaco E, Foley K, Varker H, et al. Prescribing patterns of oral antineoplastic therapies observed in the treatment of patients with advanced prostate cancer between 2012 and 2014: results of an oncology EMR analysis. Clin Ther. 2016;38:1817–1824.
