Abstract
Objectives: The aim of this study was to test choline-phosphate cytidylyltransferase-α (CCT-α) protein as a biomarker for neoadjuvant cisplatin chemotherapy response in a bladder tumor setting.
Materials and methods: A total of 238 patients with T2–T4 bladder cancer enrolled into two prior randomized trials comparing neoadjuvant cisplatin-based chemotherapy (NAC) plus cystectomy with cystectomy only (no-NAC) were used as discovery and validation cohorts. Protein expression was determined with immunohistochemistry and assessed with Histo (H)-scoring.
Results: In the discovery cohort, comprising 61 patients, the survival ratio after NAC treatment for CCT-α-negative patients was significantly increased (p = 0.001) while there was no survival advantage in the CCT-α-positive patient group. Similarly, in the validation cohort with 177 patients, NAC treatment improved survival only in the CCT-α-negative group (p = 0.006). Although there was a tendency for a good NAC response with negative CCT-α status, the interaction variable between biomarker and treatment was not significant (p = 0.24). In the cystectomy-only group, patients with positive CCT-α expression had a better survival than CCT-α-negative patients. This prognostic effect of CCT-α expression remained significant after adjusting for well-known prognostic factors in a multivariate analysis. In a pooled database of both patient data sets, multivariate analyses showed CCT-α status as an independent factor for overall survival (p = 0.018; hazard ratio = 1.80, 95% confidence interval 1.11–2.93).
Conclusion: CCT-α status was not predictive of outcome of NAC response; however, in the control group with cystectomy only it was found to have prognostic value.
Introduction
The current standard of care for muscle-invasive bladder cancer is radical cystectomy preceded by cisplatin-based neoadjuvant chemotherapy (NAC) [Citation1,Citation2]. NAC should be recommended to all eligible patients undergoing cystectomy. The expected overall survival benefit after NAC is approximately 5–8% [Citation2,Citation3], leaving the majority of patients with a potential risk of experiencing toxic side-effects of chemotherapy and delayed cystectomy unnecessarily. It is thus vital to use pretreatment predictive biomarkers to identify appropriate patients who should receive NAC.
The main mechanism of action of cisplatin and its derivatives is cancer cell death caused by DNA damage represented by cross-links and DNA adducts. The pathophysiological structures and the proteins involved in DNA damage repair can affect the action of cisplatin and are thus rational biomarker candidates [Citation4]. The nucleotide excision repair pathway with the excision repair cross-complementation group 1 enzyme (ERCC1) is the primary DNA repair mechanism, recovering platinum–DNA adducts from genomic DNA [Citation5].
Previously, ERCC1 expression in bladder tumor cells has been associated with improved prognosis in bladder cancer [Citation6]. In addition, previous results by the present group suggested that ERCC1-negative tumors respond to neoadjuvant cisplatin-based chemotherapy, whereas patients with ERCC1-positive tumors receive only a minimal benefit [Citation7]. The most documented antibody for recognizing ERCC1 protein is 8F1 antibody. There is evidence showing ERCC1 is not the principal antigen recognized by 8F1 antibody in human tissue [Citation8]. In fact, 8F1 antibody also targets an unrelated nuclear protein, choline-phosphate cytidylyltransferase-α (CCT-α) [Citation9], an important enzyme involved in phosphatidylcholine biosynthesis, nuclear membrane expansion, cellular proliferation and RAS signaling activation in response to growth factor-induced mitogen signaling [Citation10]. In lung cancer, the prognostic and predictive properties identified by 8F1 antibody were claimed to be related also to CCT-α protein expression and not only to ERCC1 [Citation11].
The hypothesis in this study was that CCT-α expression may demonstrate a correlation with NAC response and be valuable as a predictive marker.
Materials and methods
Study design and participants
A Nordic collaborative group assessed the effectiveness of chemotherapy prior to cystectomy in two consecutive trials [Citation3]. The studies included patients diagnosed with T1G3, T2–T4aNXM0 urothelial bladder cancer between 1985 and 1997. All patients were randomized to cystectomy with or without NAC. Today, NAC is not routinely used for T1G3 tumor disease going into radical cystectomy. To prevent the possible influence on survival analyses, T1G3 patients (n = 3) were removed from these data sets. As this was a new biomarker in bladder cancer research, the material was subsequently subdivided into a validation and a discovery data set according to trial inclusion.
The intended chemotherapy for patients included in the first trial was two cycles of cisplatin 70 mg/m² i.v. and doxorubicin 30 mg/m² i.v. All patients in the experimental arm and in the control arm would receive preoperative irradiation consisting of 4 Gy daily for five consecutive days. The tumor tissues of these patients from the first Nordic trial were used for the discovery set.
In the second Nordic trial, three cycles of cisplatin 100 mg/m² i.v. and methotrexate 250 mg/m² i.v. were planned for the neoadjuvant arm. Tumor tissues of patients from the second trial formed the validation data set for the present study.
Cystectomy included a lymph-node dissection of the obturator fossa. In both trials, randomization was stratified by country. Adjuvant chemotherapy has seldom been used for the patients in this data set.
Clinical staging was determined by transurethral resection (TUR) and imaging studies, whereas pathological staging was determined by cystectomy pathology. Downstaging was defined as downstaging from clinical T2 or higher to pathological Ta, Tis or T0 stages.
For the present study, tissue specimens from TUR were obtained from the Swedish patients after ethics committee approval (reference number 2008:136). The selection process is shown in . The survival analyses were conducted on an intention-to-treat basis.
Procedures
Representative tissue areas from formalin-fixed and paraffin-embedded materials were identified by a pathologist (CB) and two cores (1 mm) were obtained from these areas. The tissue microarrays were constructed with an automated instrument, ATA-27 (Beecher Instruments, Sun Prairie, WI, USA). Automated immunohistochemistry was performed, as previously reported [Citation12], with an Autostainer 480® instrument (Lab Vision, Fremont, CA, USA). In brief, the tissue slides were incubated for 30 min with the rabbit polyclonal antibody CCT-α, HPA035428 1/75 (Sigma-Aldrich, St Louis, MO, USA) before detection of the protein expression with a dextran polymer visualization system (UltraVision LP HRP polymer®; Lab Vision).
Outcomes
Staining was evaluated by a pathologist (MA) blinded to clinical and outcome data. The extent of nuclear staining in the tumor was scored as the number of cells stained: 0 to <20% (score 1), 20–50% (score 2), > 50–75% (score 3) or >75% (score 4). Intensity was scored as negative (score 0), weak (score 1), medium (score 2) or high (score 3). The product of the intensity score and the extent score was calculated to obtain a semi-quantitative Histo-score (H-score). Negative controls were produced with Universal Negative Control Serum (A. Menarini Diagnostics, Wokingham, UK) instead of the primary antibody.
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, USA) software and SAS version 9.4 (SAS Institute, Cary, NC, USA). When comparing qualitative and quantitative data between groups, chi-squared and unpaired t tests were used. The discovery cohort was used to search for an optimal cut-off for H-score regarding the response to NAC. One model was estimated for each potential cut-off value, choosing the one associated with the minimum p value (for the interaction) as the optimal cut-off value. The optimal cut-off value, found in the discovery data set, was validated in the second Nordic study (validation data set). Finally, models were estimated based on both studies (pooled data set). Results from the pooled data set were always adjusted for study. A value of p < 0.05 was considered statistically significant. Five-year overall survival (OS) and 5-year cancer-specific survival (CSS) curves were constructed according to Kaplan–Meier, and differences between groups were determined by the log-rank test. The number needed to treat was calculated with GraphPad Prism version 6 (GraphPad Software, La Jolla, CA, USA).
Lastly, to evaluate the H-scores’ ability to indicate the likelihood of benefit from NAC treatment, the patients were classified as CCT-α positive or CCT-α-negative and a Cox proportional hazard model including age, gender, T-categorization, CCT-α group, treatment, and the interaction between treatment and CCT-α group was estimated. A significant interaction indicates that the treatment effect is different for CCT-α-positive compared to CCT-α-negative patients [Citation13].
Results
The total number of the patient samples included in the study was 238. Clinicopathological data of the discovery and validation cohorts are presented in . The optimal cut-off point for H-score positivity was calculated as 8. CCT-α-negative staining was accepted as an H-score of 0–8 and positive as 9–12. Illustrations of the different nuclear staining patterns are depicted in .
Figure 2. Sample of immunohistochemical images of (a) choline-phosphate cytidylyltransferase-α (CCT-α)-negative and (b) CCT-α-positive expression (20× magnification).
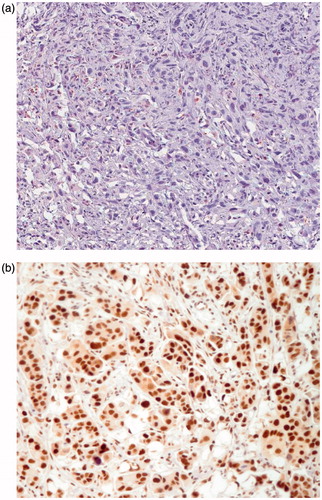
Table 1. Clinicopathological characteristics of all 238 eligible patients.
Discovery cohort
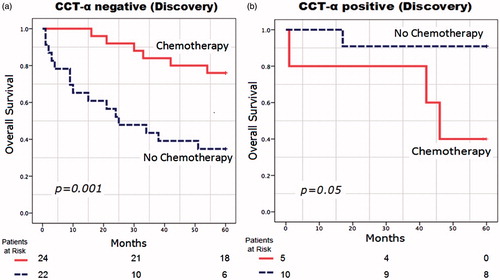
The numbers of CCT-α-negative and CCT-α-positive patients were 46 and 15, respectively. With NAC treatment, the survival ratio for CCT-α-negative patients was significantly increased (mean OS: 30 months vs 53 months, p = 0.001) (), while there was no survival advantage in the CCT-α-positive patient group (mean OS: 41 months vs 55 months, p = 0.05) (). However, CCT-α-positive patients had better survival ratios than CCT-α-negative patients when treated with only cystectomy (mean OS: 55 months vs 30 months, p = 0.005).
Validation cohort
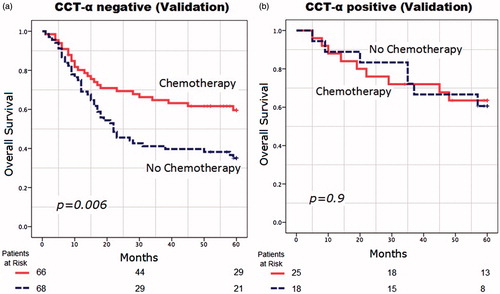
The numbers of CCT-α-negative and CCT-α-positive patients were 134 and 43, respectively. As in the discovery set, the OS was significantly better in the NAC-treated patients compared to no-NAC patients in the CCT-α-negative group (mean OS: 42 months vs 32 months, p = 0.006). No difference was found in the CCT-α-positive group (mean OS: 46 months vs 47 months, p = 0.9) (). However, evaluation of a predictive value of CCT-α status for response to NAC treatment in an adjusted analysis demonstrated that the interaction between CCT-α status and NAC administration was not significant (p = 0.24).
Figure 4. Overall survival in months from randomization in (a) choline-phosphate cytidylyltransferase-α (CCT-α)-negative patients (n = 132) and (b) CCT-α-positive patients (n = 43) enrolled in the validation data set.
In the cystectomy-only patients, CCT-α positivity was associated with a longer survival [adjusted hazard ratio (HR) 0.45, 95% confidence interval (CI) 0.20–1.00, p = 0.049], but this difference was not significant between CCT-α-positive and CCT-α-negative patients in the NAC-treated group (adjusted HR 0.87, 95% CI 0.40–1.87, p = 0.72).
Downstaging
Respective downstaging ratios after NAC for CCT-α-negative and CCT-α-positive patients were 57% and 25% (p = 0.3) in the discovery cohort and 42% and 39% (p = 0.9) in the validation cohort.
In the pooled data set of all 238 patients, no significant difference was noted in biomarker distribution between T-categories (T2/T3–4: p = 0.3). CCT-α was associated with survival when adjusting for other factors (adjusted HR 1.80, 95% CI 1.11–2.93, p = 0.018) (). Similar results were recorded with CSS calculations (data not shown).
Table 2. Multivariate analysis of overall survival in the pooled data set of all 238 patients.
The median H-score for CCT-α staining was 6. All of the survival calculations were also performed using the H-score cut-off point as 6, which gave similar survival results (data not shown).
Discussion
To the authors’ knowledge, this is the first study to evaluate the predictive effect of CCT-α expression in bladder cancer patients. The hypothesis was that CCT-α status might be predictive for NAC response in bladder cancer. Although survival analyses demonstrated an advantage for NAC treatment in CCT-α-negative patients, the interaction between CCT-α expression and NAC treatment was not significant. However, patients with positive CCT-α expression had a better survival compared to negative patients in the cystectomy-only group. This prognostic effect of CCT-α expression remained significant after adjusting for well-known prognostic factors in a multivariate analysis. Overall, the results suggest that CCT-α is prognostic rather than predictive.
A known challenge in identifying biomarkers resides in the inherent design of clinical trials. Human studies are expensive and require expertise in a wide range of areas, and almost all exploratory biomarkers to date have emerged from single-arm trials [Citation14]. It is, therefore, difficult to ascertain whether the biomarker is prognostic or predictive. Even markers identified from randomized trials have emerged from secondary analyses, and require independent validation. In this type of study, specimens that are collected, processed and archived during the course of a prospective trial are analyzed retrospectively to test the clinical utility of a tumor marker. Our biobank has this design and is thus suitable for the validation of candidate biomarkers. Another advantage compared to other studies is that the tumor specimens were obtained from the diagnostic resection.
There have been several suggestions regarding the function of CCT-α [Citation10,Citation11]. However, not all functions have been fully explored or validated, and therefore the link to prognostic effects of CCT-α is currently difficult to explain based on its suggested functions. Further studies must be performed to demonstrate the relation between the function and prognostic impact of CCT-α.
Several limitations of this investigation should be noted. Concerning the material, the trials used different chemotherapy combinations that are not considered standard today. However, both trials were included in the meta-analysis that is the basis for recommendations in the current guidelines, and none of the newer combinations has been proven to be more effective than those used earlier. Furthermore, in the first trial, preoperative radiotherapy was given to all patients, as was routine at that time. In the multivariate analysis the trial is included to control for these discrepancies. Other limitations are the limited lymph-node dissection stipulated at the time and the exclusion of the non-Swedish specimens owing to ethical policy issues, but as the trials were stratified by country the risk of confounding in this respect is minimal. The survival benefit was greater in the biobank material than in the original combined trials. When comparing stages (T2/3–4) between the original material and the biobank subset, more advanced tumors were found in the latter. The reason for this is probably that with smaller tumors it was often the case that no histological allocation material was left for immunohistochemical analyses, and thus they were also excluded when the tissue microarray was constructed (). This increased the risk that smaller tumors, possibly with better prognosis, were left out, whereas larger tumors were included for evaluation in this study.
Another limitation is that the power of the current study was insufficient to rule out the predictive value of CCT-α status. Therefore, these results need to be validated in a larger cohort before the final conclusion of CCT-α as a factor lacking predictive information can be made.
The results demonstrate novel findings that CCT-α expression in urinary bladder cancer has prognostic impact but possibly no predictive value for cisplatin-based chemotherapy response. Further studies are needed to confirm these results.
Acknowledgements
We are greatly indebted to pathologists Christer Busch (CB) and Maysaa Abdulsalam Abdulla (MA) for evaluating the tumor tissue for the cohorts and annotating the immunohistochemical stainings. We also thank the members of the Nordic Urothelial Cancer Group for their contribution to follow-up and collection of the cancer material. In addition, the statistician Lisa Wernroth is gratefully acknowledged for skillful assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866.
- Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205.
- Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45:297–303.
- Mullane SA, Werner L, Guancial EA, et al. Expression levels of DNA damage repair proteins are associated with overall survival in platinum-treated advanced urothelial carcinoma. Clin Genitourin Cancer. 2016;14:352–359.
- Rahn JJ, Adair GM, Nairn RS. Multiple roles of ERCC1-XPF in mammalian interstrand crosslink repair. Environ Mol Mutagen. 2010;51:567–581.
- Klatte T, Seitz C, Rink M, et al. ERCC1 as a prognostic and predictive biomarker for urothelial carcinoma of the bladder following radical cystectomy. J Urol. 2015;194:1456–1462.
- European Association of Urology (EAU). European Association of Urology (EAU) 29th Annual Congress: Abstract 122. April 12, 2014.
- Niedernhofer LJ, Bhagwat N, Wood RD. ERCC1 and non-small-cell lung cancer. N Engl J Med. 2007;356:2538–2540.
- Bhagwat NR, Roginskaya VY, Acquafondata MB, et al. Immunodetection of DNA repair endonuclease ERCC1-XPF in human tissue. Cancer Res. 2009;69:6831–6838.
- Sugimoto H, Banchio C, Vance DE. Transcriptional regulation of phosphatidylcholine biosynthesis. Prog Lipid Res. 2008;47:204–220.
- Vaezi AE, Bepler G, Bhagwat NR, et al. Choline phosphate cytidylyltransferase-α is a novel antigen detected by the anti-ERCC1 antibody 8F1 with biomarker value in patients with lung and head and neck squamous cell carcinomas. Cancer. 2014;120:1898–1907.
- Segersten MU, Edlund EK, Micke P, et al. A novel strategy based on histological protein profiling in-silico for identifying potential biomarkers in urinary bladder cancer. BJU Int. 2009;104:1780–1785.
- Polley MY, Freidlin B, Korn EL, et al. Statistical and practical considerations for clinical evaluation of predictive biomarkers. J Natl Cancer Inst. 2013;105:1677–1683.
- Anagnostou VK, Welsh AW, Giltnane JM, et al. Analytic variability in immunohistochemistry biomarker studies. Cancer Epidemiol Biomarkers Prev. 2010;19:982–991.