Abstract
Objective: This study aimed to validate a new method for outpatient diode laser ablation of bladder tumors without sedation or pain control.
Methods: Twenty-one patients with stage Ta low-grade intermediate-risk bladder tumors underwent photodynamic-guided laser ablation of their bladder tumors and 1 month later follow-up cystoscopy with photodynamic and IMAGE1 S™-guided biopsies. Pain was measured using a visual analog scale (range 0–10). Symptoms and worries about the future disease course were calculated using the Quality of Life Questionnaire for Non-Muscle-Invasive Bladder Cancer (range 0–100, high scores indicating worse symptoms or worry). Costs of outpatient laser treatment versus inpatient conventional bladder tumor resection in the operating theatre were compared.
Results: Patients had a median of three tumors (range 1–12). The median pain score was 1.0 (range 0–7) during laser ablation. Median quality of life scores were 24 (range 0–67) for symptoms and 42 (0–100) for worry. Two patients had minor hematuria and five had dysuria after laser therapy. Five patients (24%) had new Ta low-grade recurrence within 13 months that was biopsied and laser treated. No tumors progressed. Four patients had tumors identified using photodynamic diagnosis, and two had flat low-grade dysplasia identified using IMAGE1 S SPECTRA A and B and photodynamic diagnosis, none of which was seen using white-light cystoscopy. Outpatient laser treatment could save about €140,000 per million inhabitants versus inpatient bladder tumor surgery.
Conclusion: Fluorescence-guided diode lasers provide efficient and almost pain-free treatment of low-grade urothelial cancer in conscious patients and could reduce healthcare costs.
Introduction
Approximately 55% of patients with urothelial cancer of the bladder have low- or high-grade non-invasive bladder cancer. The tumors are normally removed using transurethral resection of bladder tumors (TURBT) under general anesthesia during a 1–2 day admission to a urology ward, preceded by 1 day of preparatory procedures. Within 5 years, 30–80% of cases recur, but only 0–2% progress to invasive cancer disease [Citation1]. Thus, non-invasive bladder cancer, in particular low-grade cancer, is a chronic disease with frequent recurrences requiring repeated TURBT and long-term monitoring. Consequently, the lifetime cost of managing bladder cancer per patient is among the highest of all cancers [Citation1–4].
Substantial healthcare resources and burden on patients could be spared if new technology could move the treatment of non-invasive bladder tumors into an outpatient or office-based setting. The authors have developed a method in which a diode laser is used to devascularize and vaporize intermediate-risk stage Ta low-grade tumors in this setting, without the use of sedation [Citation5]. As sedation is not used, the patient can leave the outpatient department immediately after the endoscope (cystoscope) has been removed. This laser destruction of bladder tumors (LDB) appears to be less painful than conventional outpatient cauterization and can therefore be used to treat larger and more numerous tumors than cauterization.
The aim of the present prospective study was to evaluate the safety, tolerability, efficacy and economic impact of outpatient LDB guided by photodynamic diagnosis (PDD) in the management of patients with recurrent Ta low-grade bladder tumors which have a low risk of disease progression.
Materials and methods
The study (ClinicalTrials.gov identifier: NCT02738827) was approved by the regional ethical committee (H-15005804) and the Danish Data Protection Agency (BFH-2015-054, I-Suite no.: 03964), and the Declaration of Helsinki (October 2013) was followed.
Patients
The study involved 21 patients (median age 66 years; range 52–89 years) who had Ta low-grade intermediate-risk bladder tumors identified during outpatient surveillance by flexible cystoscopy, where simultaneous biopsy verified the Ta low-grade histology. Patients who qualified for the study and gave written consent were booked for LDB within 1 week, and follow-up cystoscopy and biopsy 1 month after LDB to identify potential remnant tumor tissue. Inclusion criteria were: recurrent Ta low-grade urothelial bladder tumor; tumor size not larger than 1.5 cm, as larger tumors are difficult to treat through the small flexible cystoscopes; and no more than 15 tumors in the bladder. Exclusion criteria were: ongoing use of anticoagulants and macroscopic hematuria, as bleeding limits the view through the cystoscopes; pregnancy or breastfeeding; expected poor compliance; and age less than 18 years.
Procedures
Both LDB and follow-up cystoscopy were fluorescence guided using PDD (Hexvix®; Photocure, Oslo, Norway) to optimize the diagnosis of tumor tissue () [Citation6]. A new commercial technology, IMAGE1 S™ (Karl Storz, Tuttlingen, Germany), was used during the follow-up cystoscopy to assess its potential value in the diagnosis of bladder tumor recurrences after LDB. IMAGE1 S uses high-performance real-time processing, without the need for an exogenous photosensitizer, to improve the visualization of bladder details ().
Figure 1. Bladder tumor stage Ta, low grade, illuminated with (A) white light and (B) blue light (photodynamic diagnosis) by flexible cystoscopy before laser destruction of bladder tumor (LDB). (C) Mucosal lesions after LDB; the laser fiber can be seen on the right side of the picture.
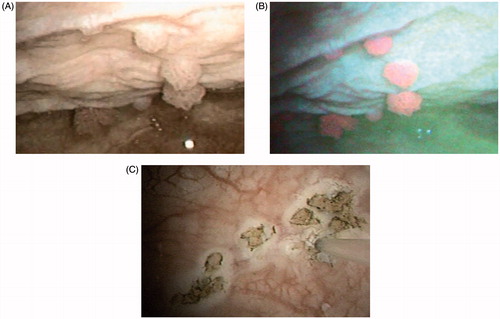
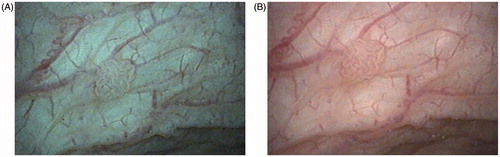
Figure 2. Bladder tumor stage Ta, low grade, examined by flexible cystoscopy using IMAGE1 S: (A) SPECTRA A; (B) SPECTRA B.
For LDB, 50 ml hexaminolevulinate (Hexvix; Photocure, Oslo, Norway) and 20 ml lidocaine anesthetic (20 mg/ml) were instilled into the bladder through a LoFric catheter Ch 12 (Astra Tech, Mölndal, Sweden). Ibuprofen 600 mg and acetaminophen 1 g were also offered as oral pain treatment. One hour later, LDB was performed using a flexible videocystoscope (11272 VPI, D-LIGHT C-LIGHT source; Karl Storz, Tuttlingen, Germany). The bladder was first inspected in white light while the urine was replaced with sterile water by irrigation of the bladder twice through the cystoscope, as urine hinders visualization during PDD by releasing green autofluorescence when illuminated by blue light [Citation7]. Then, the illumination light was switched from white to blue light and PDD was performed as blue-light cystoscopy. The locations of tumors observed in each modality were recorded on a bladder map so that laser-treated areas could be biopsied 1 month later for assessing tumor clearance after LDB.
Through the working channel of the flexible cystoscope, a laser fiber was introduced into the bladder. Laser treatment was performed with a 980 nm diode laser, 220 V, with a green 532 nm aiming beam and a front-firing 400 µm 0.22 numerical aperture bare laser fiber (Leonardo Laser, DUAL 45; Biolitec Biomedical Technology, Jena, Germany). The laser power was set to 8–15 W, with a pulse duration of 10 ms at 10 ms intervals. It was intended not to have contact between the fiber tip and the target.
Urine cytology or other urine tests were not obtained as these are not recommended for follow-up of low-grade non-invasive bladder tumor disease in European Association of Urology (EAU) and Danish guidelines.
To make sure that all tumor tissue was removed during outpatient LDB, PDD and IMAGE1 S-guided flexible cystoscopy and bladder biopsies of laser-treated areas were performed 1 month later, thus assessing the efficacy of laser therapy in terms of residual tumor rate. Biopsies were taken from previously mapped and laser-treated tumor sites, and images of the laser-treated area during PDD and IMAGE1 S examination were recorded on video. The bladder was first inspected in white light, then using five IMAGE1 S modalities (CLARA, CHROMA, CLARA/CHROMA, SPECTRA A and SPECTRA B) and eventually by PDD. This sequence was chosen as it is known that PDD is superior to white-light cystoscopy in identifying bladder tumors. Simultaneous evaluation of PDD and IMAGE1 S is not possible as the two modalities are not commercially available in the same flexible cystoscope.
Patients were given adjuvant intravesical chemotherapy instillations, according to guidelines.
Assessments
Pain experienced during LDB or cystoscopy was measured on a visual analog scale (VAS) from 0 to 10. Postprocedure symptoms were measured using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire for Non-Muscle-Invasive Bladder Cancer (QLQ-NMIBC24). The QLQ-NMIBC24 has been validated in Danish patients after TURBT under general anesthesia and has a ‘symptom score’ for lower urinary tract symptoms (LUTS) and a ‘worry score’ for concerns about the future course of the disease [Citation8]. The range of both scores is 0–100, with higher scores indicating worse LUTS or worry. The VAS was filled out immediately after the laser procedure, whereas the QLQ-NMIBC24 was completed on day 7 after LDB. A research nurse called patients on day 5 to remind them to complete the questionnaire and to ask about adverse events. After the follow-up cystoscopy, patients were asked whether they preferred the inpatient or the outpatient procedure for future tumor recurrences.
Bladder lesions were classified according to Union for International Cancer Control/American Joint Committee on Cancer stages [Citation9] and WHO 2004 grades [Citation10].
To assess the economic impact of outpatient LDB, public reimbursement of inpatient TURBT and outpatient LDB based on diagnosis-related groups [Citation11] were compared. Reimbursement in Denmark is based on the Danish National Healthcare classification system [Sundheds-vaesenets Klassifikations System (SKS)], which follows the International Classification of Diseases, 10th revision, but is adapted to Danish costs of surgical procedures. The SKS codes used were KZXF45 for the PDD procedure, KKCD32 for TURBT and DD303 for Ta low-grade bladder tumor [Citation12]. A code for outpatient LDB does not exist. Instead, the code for outpatient cystoscopy and biopsy was used, and the cost of a laser fiber was added. The number of TURBT procedures was obtained from the Danish bladder cancer registry [Citation13].
Statistical analysis
As a basic level of efficacy, the proportion of patients having unbearable discomfort and side-effects hindering LDB should be small. With a sample size of 21 and if the proportion of patients with unbearable discomfort is 25%, the hypothesis that less than 5% of the patients have unbearable discomfort can be rejected with a power of 80%, using an exact binomial test. The observed proportion of patients with discomfort was determined with a 95% exact confidence interval (CI). Calculations were performed in R version 3.3.1.
Results
Patients had a median of three recurrent tumors each (range 1–12; tumor size 5–15 mm). The median duration of cystoscopic examination and laser treatment was 29 min (range 19–45 min), and the median delivered laser effect was 700 J (range 103–1840 J).
All patients had LDB performed as scheduled without significant discomfort. The hypothesis that less than 5% of the patients experience unbearable discomfort could not be rejected (p = 1.00, 95% CI 0–16%). The median VAS score for pain was 1.0 (range 0–7; only one patient recorded level 7) during LDB and 0.3 (range 0–4) during follow-up cystoscopy and biopsy. On the QLQ-NMIBC24 recorded after 7 days, the median symptom score was 24 (range 0–67) and the median worry score was 42 (range 0–100). Two patients (10%) had minor hematuria for 24 h and five patients (24%) experienced dysuria without bacteriuria for up to 4 days; none of these symptoms required treatment, as no urinary infections were recorded. No other symptoms were recorded. All patients preferred to have outpatient LDB instead of inpatient TURBT for future recurrences.
At 1 month cystoscopy, 19 (90%) of the 21 patients had no tumor tissue in biopsies from laser-treated areas, whereas two patients had remnant flat low-grade dysplasia. Over a median follow-up of 14 months (range 12–16 months), five patients (24%) experienced Ta low-grade recurrences, which were small (1–3 mm) and could be immediately biopsied and treated by laser. No patients had disease progression.
During LDB, PDD identified tumors that were not seen on white light in four patients (19%). During follow-up cystoscopy at 1 month, IMAGE1 S SPECTRA A and SPECTRA B settings gave images that appeared to accentuate irregularities in the mucosa and the contrast of vessels, more than SPECTRA CLARA/CHROMA and CLARA did. SPECTRA A and B identified flat low-grade dysplasia in two patients (10%), which was also seen on PDD but not on white light or CLARA/CHROMA or CLARA settings. The median duration of PDD, IMAGE1 S and biopsy was 16 min (range 9–34 min).
Reimbursement for inpatient management of Ta low-grade bladder tumor, i.e. hospital admission and PDD-guided TURBT in the operating room, is €2508. Using the code for outpatient PDD-guided cystoscopy and biopsy (€771) and the price of a laser fiber (€135), reimbursement for PDD-guided LDB was €906. Thus, the outpatient procedure offered a €1602 cost reduction compared with inpatient management. According to the Danish national bladder cancer registry, about 1944 TURBT procedures are performed per year in Denmark due to recurrent Ta low-grade tumors. Based on clinical experience, the authors estimate that 25% of these procedures (approximately 486 TURBTs for Ta low-grade) are carried out for tumors less than 1.5 cm in size and fewer than 15 in number. These can therefore be removed with LDB in the outpatient department. With a Danish population of 5.5 million, this equates to approximately 88 procedures per million inhabitants that could be performed in the outpatient department, achieving estimated direct cost savings of about €140,000 per million inhabitants.
Discussion
This study indicates that outpatient laser treatment is safe and causes little discomfort for patients with recurrent intermediate-risk Ta low-grade bladder tumors. As no sedation is used and symptoms are sparse, all patients were able to continue daily activities immediately after the procedure. Previous reports on outpatient removal of bladder tumors under local anesthesia are limited to the treatment of fragile elderly patients and patients with multiple comorbidities, for whom general anesthesia presents a high risk [Citation14]. This article proposes a treatment modality that may be used for any patient with a moderate tumor burden, and not only fragile patients. The intention is that the tolerability of LDB will allow patients to leave the outpatient department directly after the procedure and return to their normal routine.
This study was limited to recurrent tumors to ensure that patients had a reliable diagnosis of non-invasive low-grade bladder cancer after conventional inpatient TURBT, so the patient was not jeopardized by treatment of invasive aggressive disease. Accordingly, primary tumors should not be handled with LDB.
Furthermore, the low grade of tumor recurrence in the present study was confirmed on biopsy before the laser procedure to avoid high-grade disease and its higher risk of progression. Other investigators have reported recurrence rates up to 73% 3 months after inpatient yttrium aluminum garnet (YAG) laser treatment of non-muscle-invasive bladder cancer, including T1 disease [Citation15]. A 6% progression rate in the 2 years after outpatient holmium:YAG laser treatment has been reported in patients with T1 high-grade tumors, compared with no progression in those with Ta low-grade tumors [Citation16]. In the absence of substantial evidence on the efficacy of outpatient laser treatment of bladder cancer, only Ta low-grade disease was treated in this study, because the risk of progression is low in these tumors.
This is the first report on the efficacy and usability of diode lasers in the treatment of bladder tumors. A 980 nm diode laser was chosen because of its significant optical absorption coefficient for hemoglobin (50 cm−1), which is sufficient to heat and ensure coagulation of blood vessels in tumors, thereby avoiding bleeding obscuring visualization [Citation5]. Coagulation of blood vessels also induces ischemia and subsequent exfoliation of remnant tumor tissue. Furthermore, a low absorption coefficient in water (0.3 cm−1) makes deep tissue penetration possible, as measured up to 4.5 mm, which appears sufficient for influencing the entire tumor base and stalk in exophytic tumors of this size [Citation17].
The 24% recurrence rate in this study (five out of 21 patients over a median of 14 months) is similar to that reported after treatment with holmium lasers and to the risk tables in EAU guidelines [Citation14,Citation18–20]. Three of these recurrences appeared after 5 months and may have been residual tumors after insufficient laser treatment, like the two cases of remnant flat low-grade dysplasia in laser-treated tumor areas identified at 1 month. These patients typically had either many tumors or large tumors at the initial LDB, which need expertise to eradicate with laser treatment. The recurrence rate would be expected to decrease with increasing experience with the technique. In the meantime, residual tumor tissue or small recurrences are easy to remove in the outpatient department, without referral to the urology ward for TURBT. In all cases of recurrence in this study, the pathological lesions were immediately removed by laser therapy.
The benefits of PDD over white light to guide flexible cystoscopy in the outpatient setting have been described [Citation21], but, there are only two reports of bladder examination with IMAGE1 S [Citation22,Citation23]. The advantage of IMAGE1 S is its instant use without preceding procedures. Although the number of patients examined using IMAGE1 S in this study is low, this experience suggests that further investigation of the value of SPECTRA A and B, and whether they facilitate immediate diagnosis of bladder tumors, is warranted.
The impact of LDB on patients, measured by the EORTC QLC-NMIBC24, shows significant differences from TURBT. In a population of similar patients undergoing inpatient TURBT, the authors measured a symptom score of 45, which is double the score in the LDB patients, indicating that TURBT is more traumatizing than LDB. However, the worry score in the TURBT study was 40 and thus similar to the score in the present study, indicating that outpatient management with LDB is not yet sufficient to alleviate patients’ concerns about the future course of the disease [Citation8].
The economic benefits of transferring inpatient TURBT to outpatient LDB are substantial. In addition to the calculated cost reduction, operating room capacity is spared, so it can be used for alternative surgical procedures. Working days may also be spared and societal and personal costs thus reduced as patients are able to return to their jobs immediately after LDB. However, if LDB replaces day-care surgical procedures, the cost benefit may be slightly lower than estimated owing to the lower cost of day care than inpatient procedures. For patients, outpatient LDB is easier and much less demanding, and, according to this study, is preferred by patients over inpatient TURBT. Based on the results of the present study, the method is now under evaluation in a randomized controlled trial (ClinicalTrials.gov identifier: NCT02886026).
Disclosure statement
G. G. Hermann has provided services as a speaker in meetings funded by Photocure. The other authors have no competing financial interests.
Additional information
Funding
References
- van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009;56:430–442.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403.
- Sangar VK, Ragavan N, Matanhelia SS, et al. The economic consequences of prostate and bladder cancer in the UK. BJU Int. 2005;95:59–63.
- James AC, Gore JL. The costs of non-muscle invasive bladder cancer. Urol Clin North Am. 2013;40:261–269.
- Hermann GG, Mogensen K, Lindvold LR, et al. Office-based transurethral devascularisation of low grade non-invasive urothelial cancer using diode laser. A feasibility study. Lasers Surg Med. 2015;47:620–625.
- Hermann GG, Mogensen K, Carlsson S, et al. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: a randomized two-centre study. BJU Int. 2011;108:E297–E303.
- Lindvold LR, Hermann GG. An optical method for reducing green fluorescence from urine during fluorescence-guided cystoscopy. Methods Appl Fluoresc. 2016;4:0450002.
- Mogensen K, Christensen KB, Vrang ML, et al. Hospitalization for transurethral bladder resection reduces quality of life in Danish patients with non-muscle-invasive bladder tumour. Scand J Urol. 2016;50:170–174.
- Sobin LH, Wittekind C, editors. TNM classification of malignant tumors. 6th ed. Hoboken (NJ): John Wiley & Sons; 2002.
- World Health Organization (WHO). WHO classification of tumours: pathology & genetics. Tumours of the urinary system and male genital organs. Lyon (France): IARC Press; 2004.
- Busse R, Geissler A, Quentin W, et al. editors. Diagnosis-related groups in Europe. European Observatory on Health Systems and Policies Series. New York (NY): McGraw Hill/Open University Press; 2011.
- Sundhedsdata Styrelsen. Interaktiv DRG.2017. Available from: http://drgservice.ssi.dk/grouper/Modules/Home/
- DBCR 2010 årsrapport. Dansk BlaereCancer Register & Forskningscenter for Forebyggelse og Sundhed. [Internet] 2012. Available from: http://ducg.dk/dablaca-blaerecancer/aarsrapporter/
- Wong KA, Zisengwe G, Athanasiou T, et al. Outpatient laser ablation of non-muscle-invasive bladder cancer: is it safe, tolerable and cost-effective? BJU Int. 2013;112:561–567.
- Kramer MW, Wolters M, Cash H, et al. Current evidence of transurethral Ho:YAG and Tm:YAG treatment of bladder cancer: update 2014. World J Urol. 2015;33:571–579.
- Syed HA, Talbot N, Abbas A, et al. Flexible cystoscopy and holmium: yttrium aluminum garnet laser ablation for recurrent nonmuscle invasive bladder carcinoma under local anesthesia. J Endourol. 2013;27:886–891.
- Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev. 2003;103:577–644.
- Johnson DE. Use of the holmium:YAG (Ho:YAG) laser for treatment of superficial bladder carcinoma. Lasers Surg Med. 1994;14:213–218.
- Xishuang S, Deyong Y, Xiangyu C, et al. Comparing the safety and efficiency of conventional monopolar, plasmakinetic, and holmium laser transurethral resection of primary non-muscle invasive bladder cancer. J Endourol. 2010;24:69–73.
- Zhong C, Guo S, Tang Y, et al. Clinical observation on 2 micron laser for non-muscle-invasive bladder tumor treatment: single-center experience. World J Urol. 2010;28:157–161.
- Hermann GG, Mogensen K, Toft BG, et al. Outpatient diagnostic of bladder tumours in flexible cystoscopes: evaluation of fluorescence-guided flexible cystoscopy and bladder biopsies. Scand J Urol Nephrol. 2012;46:31–36.
- Kamphuis GM, de Bruin DM, Brandt MJ, et al. Comparing image perception of bladder tumors in four different Storz professional image enhancement system modalities using the iSPIES app. J Endourol. 2016;30:602–608.
- Kamphuis GM, de Bruin DM, Fallert J, et al. Storz Professional Image Enhancement System: a new technique to improve endoscopic bladder imaging. J Cancer Sci Ther. 2016;8:71–77.
