Abstract
Objective: The aim of this study was to assess whether sclerosis in histology following bulbar urethroplasty is a predictive factor for failure of surgery.
Materials and methods: Resected stricture specimens from 45 patients undergoing open urethroplasty with excision and anastomosis were collected prospectively during 2011–2014. Histopathological characteristics, including fibrosis (grade I–III), inflammation and sclerosis, were evaluated using different routine staining. These specimens were compared to normal urethral resection specimens from patients undergoing sex-correction surgery. The uropathologist who conducted the analyses was blinded to the study design.
Results: The outcomes of the histological classifications were as follows: 19 patients had grade I fibrosis, of whom three had failures; 13 patients had grade II fibrosis, without any failures; and the most severe fibrosis, grade III, including sclerosis, was found in 13 patients (11 with sclerosis), with failure in eight. Sclerosis was a significant risk factor for restricture when comparing patients with sclerosis and those without sclerosis, and likewise when adjusting for age, inflammation and stricture length.
Conclusion: Histological findings of sclerosis in the resected urethral stricture specimen indicate a significantly higher risk for restricture after urethroplasty surgery.
Introduction
Processes that injure the urethral epithelium, or the underlying corpus spongiosum, may bring about scar formation, which in turn can give rise to a urethral stricture. Contraction of this scar reduces the urethral lumen to such a degree that voiding symptoms eventually may occur. Underlying reasons include various kinds of insults such as instrumentation, infection, catheterization and perineal trauma [Citation1].
Inflammatory mediators and signalling molecules appear to be of importance in the scar formation. Transforming growth factor-β1 (TGF-β1) has been reported to play a significant role in fibrotic diseases [Citation2,Citation3]. In addition, immunoglobulin G4 (IgG4)-related disease is a fibrous inflammatory condition that can affect essentially any organ and consists of infiltrating IgG4-positive plasma cells [Citation4]. Targeted treatment approaches in this immunological field may be feasible for treating fibrosis in urethral strictures. Excessive fibrosis is also characterized by an increase in collagen type I and a decrease in collagen type III [Citation5]. Hence, it appears that the wound-healing process in stricture disease is almost invariably associated with inflammation. Migration of fibroblasts and collagen to the injured area causes fibrosis of different degrees, which may involve sclerosis [Citation5]. However, the exact mechanisms that bring about such fibrotic reactions remain to be elucidated. A firm correlation between the degree of fibrosis and the degree of narrowing of the urethral lumen has, to the authors’ knowledge, never been reported. In addition, the question as to whether the degree of fibrosis in scar tissue can predict outcome after urethroplasty has not been addressed. The aims of this study were to investigate the inflammatory responses in resected urethral stricture specimens and to determine whether sclerosis is a predictive marker for urethroplasty failure.
Materials and methods
Patient cohort selection
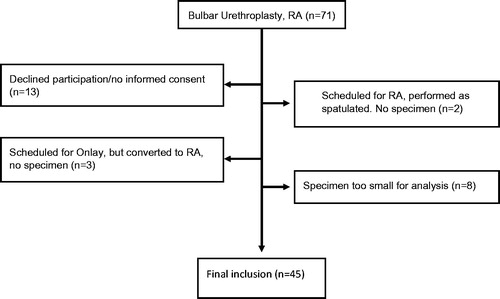
This series comprises 45 patients, prospectively and consecutively recruited, with bulbar urethral strictures subjected to urethroplasty with resection and end-to-end anastomosis during the period December 2011 to December 2014 at Sahlgrenska University Hospital, Gothenburg, Sweden. Out of the initial 71 patients, 26 were excluded for various reasons (). The removed diseased urethral segments were analysed by a uropathologist. The majority of the strictures in the present study were single site strictures (in a few cases there were two bulbar strictures in very close proximity to each other and they were, in all cases, resected in one piece) and the ends of the stricture segments were assessable urethroscopically or radiologically. The research protocol was approved by the University of Gothenburg regional ethical review board (permit no. 663-11) and the patients provided informed consent before the urethroplastic surgery.
Follow-up
The patients were followed at 3 months, 1 year and 2 years postoperatively and always on request. Once the patients had experienced 2 years of satisfactory outcome, they were discharged from the follow-up program and instructed to contact the clinic if micturition problems should develop. Failure was defined as the appearance of a new stricture necessitating a surgical intervention, such as dilatation, internal urethrotomy or a new urethroplasty. Restricture was diagnosed at cystoscopy during follow-up, in combination with the patient’s symptoms.
Tissue handling
Visible scar tissue was excised and an excision with anastomosis was performed as previously described [Citation6]. The specimens were fixed in 10% neutral-buffered formalin, dehydrated and embedded in paraffin. Sections measuring 4 µm were stained using three different techniques (hematoxylin–eosin, Van Gieson and Masson’s trichrome), with each block analysed in a standard way. The diagnosis was evaluated by a uropathologist, who was blinded to the study design. The degree of fibrosis was classified as mild, moderate or severe, essentially according to a scheme described previously in the literature [Citation7,Citation8]. These specimens were compared to two resection specimens containing normal urethrae from patients who had been subjected to sex-correction surgery, i.e. penectomy/urethrectomy.
Samples of normal urethra
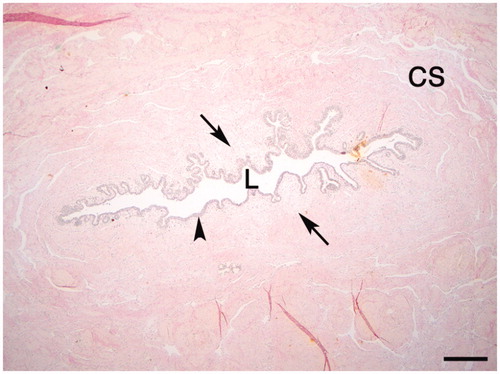
The normal male urethra is defined as a layer of pseudo-stratified columnar epithelium or squamous epithelium, direct into the basement membrane, underlined by loose connective tissue in the mucosa, with macrophages and fibroblasts present in various numbers. Further from the lumen the connective tissue, containing fibroblasts and extracellular matrix with collagen and glycoproteins, increases, and smooth muscular bundles appear with vessels and vascular sinusoids of the corpus spongiosum [Citation9,Citation10]. Further out from the lumen, massive collagen bundles are apparent ().
Fibrosis
Mild fibrosis (grade I) in the urethra was defined as loose connective tissue with small subepithelial areas that contained higher levels of collagen and fibroblasts but did not display a thickening of the wall (). Moderate fibrosis (grade II) displayed wider areas with subepithelial collagen. Loose connective tissue could be seen in the spongiosum and the fibrosis extended deeper into the wall of the urethra (). In severe fibrosis (grade III), loose vascular subepithelial tissue was lacking. More widespread and more deeply extending fibrosis was also noted, as well as a thickening of the wall of the urethra. Sclerosis, which is a thickening and hardening of the fibrosis and has a typical appearance histologically, was found in grade III fibrosis ().
Figure 3. (A–C) Urethral sections with fibrosis and sclerosis classified as grades I–III: (A) grade I; (B) grade II; (C) grade III. L, lumen. F, fibrosis. Sc, Sclerosis. Arrowheads, inflammation; arrow; germinal centre formation. (Van Gieson stain.) (D–F) Urethral tissue sections of grade III with sclerosis in (D) H&E and (E) Masson’s trichrome; (F) higher magnification of (E). Scale bars = 500 μm.
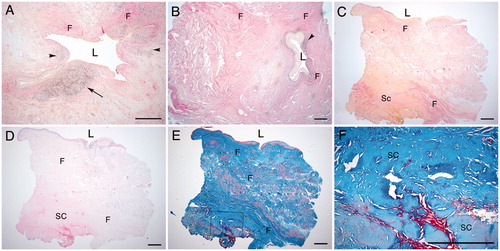
In some cases, erosion and ulceration appeared in the luminal urothelium/squamous urothelium, with underlying granulation tissue, angiogenesis and fibroblast proliferation leading to fibrosis, especially in cases with grade I fibrosis.
Inflammation
Inflammation was defined as an immunological defence process initiated against local tissue damage, for example an infection or a trauma. It is a complex reaction of blood vessels, certain plasma components and blood cells, such as neutrophils, leucocytes and plasma cells. There can be an acute inflammation and sometimes the immune system fights against its own cells by mistake, hence causing a chronic inflammatory disease.
Sclerosis
Sclerosis was defined as hardening or thickening of the tissue of the organ, attributed to chronic inflammation with an increase in the connective tissue, the presence of fibres and excessive formation of fibrous interstitial tissue.
Statistical analysis
Kaplan–Meier with log-rank test was used for the recurrence-free survival analysis (). A Cox regression model was fitted to estimate the hazard ratio (HR) for failure in patients with and without sclerosis adjusted for inflammation, age and stricture length (see ). Adjustments for aetiology were not possible because the majority of the aetiology was not known. A p value of <0.05 was considered to indicate statistical significance. Statistical calculations were performed with SPSS version 20 for Mac (IBM Corp., Armonk, NY, USA) and R Statistical Software version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Figure 4. Kaplan–Meier curves showing recurrence-free rates: (A) after open urethroplasty (n = 45), resection with anastomosis (RA), for bulbar stricture; (B) between fibrosis grades I–III (log-rank test; p = 0.024); and (C) comparing patients with sclerosis and those without sclerosis (log-rank test; p = 0.020).
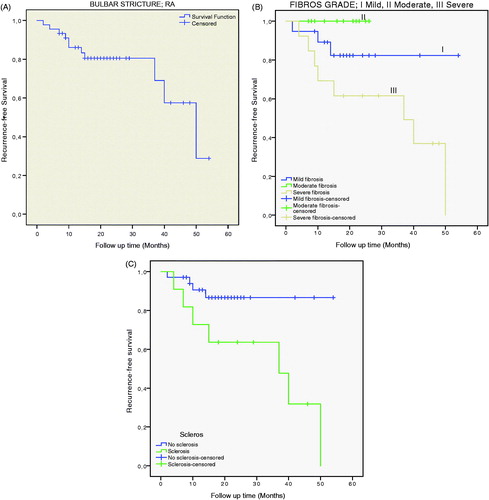
Table 1. Patients’ characteristics: age, sclerosis, failure (restricture; in need of a new intervention), inflammation, stricture length and aetiology in each grade of fibrosis.
Table 2. Multivariable Cox regression analysis of variables: age, stricture length (SL), inflammation and sclerosis.
Results
In the control specimens (N = 2), the tissue was normal without any signs of fibrosis, inflammation or sclerosis, whereas all 45 specimens following urethroplasty had fibrosis. Nineteen patients had grade I fibrosis, 13 patients had grade II fibrosis and 13 patients had grade III fibrosis.
After a median follow-up of 21 (range 7–54) months, 11 failures were identified.
The mean and median age were 41 and 36 years (range 16–75 years) (). The success rate in all 45 patients was 76% according to this study (). Success rates in grades I, II and III were 89%, 100% and 38%, respectively () (log-rank test; p = 0.024). Success rates for patients without and with sclerosis were 88% and 36%, respectively () (log-rank test; p = 0.02).
Sclerosis was clearly identified in 11 of the specimens, all in grade III fibrosis. Seven of these 11 patients, who had visible sclerosis in their resection specimens, redeveloped stricture necessitating redo surgery, whereas in the patients who lacked sclerosis (n = 34) only three had a restricture, a significant difference. The hazard ratio for failure in patients with sclerosis (HR = 3.93, 95% confidence interval 1.09–14.16; p = 0.036) was four times higher than in those without, adjusted for inflammation, age and stricture length ().
Chronic inflammation was present in the study specimens, in the form of inflammatory cells, such as lymphocytes, plasma cells and germinal centre formation (). Inflammation was observed in 14 patients, two of whom had restricture. Inflammation and restricture were evenly distributed across the three grades of fibrosis ().
Discussion
In the present series, the finding of sclerosis came out as a significant risk factor for failure after resection with anastomosis due to bulbar urethral stricture. Herein, all of the patients with sclerosis who had restricture were associated with the highest grade of fibrosis, grade III; the risk for failure was about four times higher, adjusted for inflammation, age and stricture length, when sclerosis was found in the excised specimen. This strengthens the contention that it is important to excise all the fibrotic tissue during primary surgery, to diminish the risk for restricture. During surgery, the aim is to excise all scarred tissue. However, it is impossible to ascertain that the margins are microscopically free from fibrosis. The success rate in this study (76%) was somewhat lower than would normally be seen (around 90%). One explanation for this could be that not all patients agreed to the study and signed the informed consent, while some patients signed and had an onlay instead of a resection because of too long a stricture.
The bulbar urethra is the most common location for urethral stricture and the aetiology may be idiopathic, iatrogenic, traumatic or infectious; in the report from Fenton et al., these are fairly evenly distributed [Citation11]. This is at variance with the present series, in which the majority of the patients had an idiopathic aetiology (70%). This was followed by traumatic aetiology in eight patients (17%), two of whom had a restricture, one with sclerosis and one without.
The radiological assessment used to evaluate the extent of the spongiofibrosis includes voiding cystourethrography; some even use magnetic resonance imaging (MRI) [Citation12,Citation13], reporting that MRI may add valuable information such as associated pathologies in association with the stricture [Citation14].
The authors did not have a clear histological basis or classification for the formation of the stricture and the fibrosis until the present series, in which fibrosis was defined in three grades, I–III. This study uses a proposed classification of fibrosis and sclerosis, and a recognized classification would greatly facilitate multicentre studies and comparisons between centres. Fibrosis is a general healing feature after inflammation or necrosis and may exist as pulmonary fibrosis, kidney fibrosis, scleroderma, Hodgkin’s lymphoma, cardiac fibrosis and common scar tissue in the skin after wound healing. These conditions appear to express a somewhat similar histopathological pattern [Citation15,Citation16]. Scar formation, causing a urethral stricture, is dense, hypovascular and reduced in elastic fibres, and also contains fibroblasts. The healing process in, for example, wound inflammation includes the stimulation of interleukin-13, which stimulates TGF-β1, which, in turn, stimulates fibroblasts that produce extracellular matrix and fibrosis [Citation17].
Some ways to avoid fibrosis may involve the use of fibrin glue, which causes no inflammation or fibrosis formation, according to Barbagli et al. [Citation18]. Suggestions have also been proposed to inject mitomycin C, which inhibits DNA synthesis and delays the healing process by preventing replication of fibroblasts and inhibiting collagen synthesis [Citation19]. This process can be inhibited in various ways, as described above, but will need further studies to be implemented clinically on a large scale [Citation8,Citation20–22].
This study did not find a significant risk for restricture caused by inflammation, age or stricture length. Sclerosis, as the terminal outcome of fibrosis, appeared only in grade III fibrosis, as illustrated in , with a success rate of 36%. This indicates that the success rate is low in patients with grade III fibrosis and sclerosis. However, the number of specimens studied was fairly small and follow-up times varied, which are obvious limitations. Another shortcoming is that there is no uniformly established classification concerning the severity of fibrosis. The proposed classification in the present series could be of help for future histopathological investigations of urethral stricture disease. The control specimens with normal urethra came from patients who had undergone sex-correction surgery, and their preoperative treatment with oestradiol may have had an effect on the urethra; however, to the authors’ knowledge, no evidence for this exists [Citation23,Citation24].
There are several strengths to the present study, including its prospective design, the consistent follow-up and the fact that the pathologist was blinded to the clinical outcome. The major strength with the study is the fact that sclerosis in the specimen is an indisputable and clear-cut histopathological finding that is agreed upon among surgical pathologists. Therefore, the specimens can be dichotomized between those that display sclerosis and those that do not. The clear and highly significant finding that the patients with sclerosis were strikingly overrepresented when it came to the redevelopment of stricture disease is the most important message in this study. Besides adding to the knowledge on stricture histopathology, it may serve as a valuable tool for the reconstructive surgeon, allowing for the individual tailoring of a suitable follow-up schedule based on histopathological findings in the resected specimen. Moreover, in cases of restricture, knowledge of the histopathological features at primary surgery may be helpful when deciding on which technique to choose at redo surgery. The present findings may even open up the possibility of using frozen sections during surgery, in order to elucidate the involvement of sclerosis (although diagnosing sclerosis in frozen sections may be difficult) and extension; in cases where sclerosis is found in the resection margin, excision of more tissue may be beneficial, perhaps necessitating an augmentation with onlay surgery. Finally, further studies in a larger sample size are needed to confirm that sclerosis is a risk factor for restricture and whether excessive excision of the fibrosis can diminish the risk for restricture.
In conclusion, histological findings of severe fibrosis with sclerosis in the resected urethral stricture specimen indicate a four-fold higher risk for restricture after surgery. A classification of the histopathology of the fibrosis may be helpful for further studies in the field of urethral stricture.
Acknowledgments
We are deeply thankful to Dr Gennaro Selvaggi, MD, PhD, Department of Plastic Surgery at Sahlgrenska University Hospital, Gothenburg, Sweden, for providing the specimens of normal urethra. The authors thank the photographer Ulric Pedersen, Department of Pathology, Sahlgrenska University Hospital, for the excellent microphotographs.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Lumen N, Hoebeke P, Willemsen P, et al. Etiology of urethral stricture disease in the 21st century. J Urol. 2009;182:983–987.
- El-Sakka AI, Hassoba HM, Chui RM, et al. An animal model of Peyronie's-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol. 1997;158:2284–2290.
- Border WA, Noble NA. Fibrosis linked to TGF-beta in yet another disease. J Clin Invest. 1995;96:655–656.
- Stone JH. IgG4-related disease: pathophysiologic insights drive emerging treatment approaches. Clin Exp Rheumatol. 2016;34:66–68.
- Baskin LS, Constantinescu SC, Howard PS, et al. Biochemical characterization and quantitation of the collagenous components of urethral stricture tissue. J Urol. 1993;150:642–647.
- Ekerhult TO, Lindqvist K, Peeker R, et al. Low risk of sexual dysfunction after transection and nontransection urethroplasty for bulbar urethral stricture. J Urol. 2013;190:635–638.
- Sangkum P, Yafi FA, Kim H, et al. Collagenase Clostridium histolyticum (Xiaflex) for the Treatment of Urethral Stricture Disease in a Rat Model of Urethral Fibrosis. Urology. 2015;86:647.e1–646.
- Sangkum P, Gokce A, Tan RB, et al. Transforming Growth Factor-β1 Induced Urethral Fibrosis in a Rat Model. J Urol. 2015;194:820–827.
- Singh M, Scott TM. The ultrastructure of human male urethral stricture. Br J Urol. 1975;47:871–876.
- Singh M, Blandy JP. The pathology of urethral stricture. J Urol. 1976;115:673–676.
- Fenton AS, Morey AF, Aviles R, et al. Anterior urethral strictures: etiology and characteristics. Urology. 2005;65:1055–1058.
- Barbagli G. Adherence to the right diagnostic tools for best outcomes in urethral reconstructive surgery. Eur Urol. 2006;50:424–425.
- Osman Y, El-Ghar MA, Mansour O, et al. Magnetic resonance urethrography in comparison to retrograde urethrography in diagnosis of male urethral strictures: is it clinically relevant? Eur Urol. 2006;50:587–593.
- El-Ghar MA, Osman Y, Elbaz E, et al. MR urethrogram versus combined retrograde urethrogram and sonourethrography in diagnosis of urethral stricture. Eur J Radiol. 2010;74:e193–e198.
- Yoshida Y, Nagata N, Tsuruta N, et al. Heterogeneous clinical features in patients with pulmonary fibrosis showing histology of pleuroparenchymal fibroelastosis. Respir Investig. 2016;54:162–169.
- Gallet R, de Couto G, Simsolo E, et al. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci. 2016;1:14–28.
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292.
- Barbagli G, De Stefani S, Sighinolfi MC, et al. Bulbar urethroplasty with dorsal onlay buccal mucosal graft and fibrin glue. Eur Urol. 2006;50:467–474.
- Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol. 2007;51:1089–1092.
- Liu M, Zeng X, Wang J, et al. Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Res Ther. 2016;7:63.
- Yardimci I, Karakan T, Resorlu B, et al. The effect of intraurethral dexpanthenol on healing and fibrosis in rats with experimentally induced urethral trauma. Urology. 2015;85:274 e9–213.
- Zhang K, Guo X, Zhao W, et al. Application of Wnt Pathway Inhibitor Delivering Scaffold for Inhibiting Fibrosis in Urethra Strictures: In Vitro and in Vivo Study. Int J Mol Sci. 2015;16:27659–27676.
- Gooren L. Hormone treatment of the adult transsexual patient. Horm Res Paediatr. 2005;64: 31–36.
- Trum HW, Hoebeke P, Gooren LJ. Sex reassignment of transsexual people from a gynecologist's and urologist's perspective. Acta Obstet Gynecol Scand. 2015;94:563–567.