Abstract
Objective: To describe the management of patients with hematuria in the Nordic countries in relation to bladder cancer epidemiology, especially in the context of introducing fast track pathways with the aim of proposing a common guideline.
Materials and methods: Epidemiological data on bladder cancer from each country, and the combined cancer registry, Nordcan, were analyzed. The evolution of the different national recommendations and the introduction of fast track pathways were assessed. Patients' demographics, type of hematuria and cancer detection rates were analysed if available.
Results: The crude incidence of bladder cancer has increased substantially since the 1960s, while the age standardized incidence has been stable during recent decades. The relative survival has increased in all countries, while the mortality has been stable. For those with microscopic hematuria there has been a clear trend towards less rigorous investigations. In the fast track pathways, introduced in three of five countries, about one in five patients with macroscopic hematuria had a cancer diagnosis. Data show that time to diagnosis has been reduced.
Conclusions: The number of patients with bladder cancer is increasing in the Nordic region. The introduction of fast track pathways has been important in improving the management of patients with suspicion of the disease. Our recommendation is to focus on macroscopic hematuria in the fast track pathways. Microhematuria without any symptoms should not be an indication for cystoscopy. However, urinary tract symptoms accompanied by microhematuria can still be investigated according to respective guidelines but not necessarily within fast track pathways.
Introduction
Hematuria, while being a common medical problem, is seldom analysed on a population level. Microscopic and macroscopic, with or without symptoms, is calculated to account for four consultations per thousand patients per year in primary care in the U.K. [Citation1]. Macroscopic hematuria (MaH) is the cancer alarm symptom associated with the highest risk of detecting a malignancy in the British database. Painless macroscopic hematuria was associated with 3-year positive predictive values of 7.4% in men and 3.4% in women for the diagnosis of bladder cancer [Citation1].
The clinical significance of microscopic hematuria (MiH) on the other hand is more controversial. Diagnostic guidelines vary widely, but most agree on a definition of MiH as > three red blood cells per high power microscope. Many use a positive urine dipstick as a diagnostic for MiH, as specificity has been shown to be equal to microscopy, and in Nordic area microscopy has been largely replaced by dipstick testing. Dipsticks are also used to test for diabetes, U.T.I. and proteinuria, making their use widespread. Moreover, the use of a dipstick is included in E.A.U. guidelines for several non-oncologic diseases, with recommendations from weak to strong.
In the general adult population, MiH is found in 2–7% of men and in 3–15% of women [Citation2]. Its sensitivity is reported to be 51% and specificity 78% for bladder cancer detection [Citation3]. However, most initial screening studies had insufficient power to allow conclusions to be drawn about the value of dipstick urine testing, although a more recent, relatively large, study showed that the diagnostic yield in an unselected population was too small to make population-based screening cost-effective [Citation4]. Some studies have investigated dipstick urine testing in selected high-risk groups, such as heavy smokers and individuals with environmental exposure to bladder carcinogens, but these studies have not shown that the benefits outweigh the costs [Citation5,Citation6]. A Cochrane analysis was performed to investigate whether screening for MiH could decrease morbidity and mortality [Citation7]. The conclusion was that the quality of the reported studies was too low to support any recommendation.
A symptomatic MiH diagnosis originates from a patient usually investigated for lower urinary tract symptoms (L.U.T.S.) with a positive dipstick. Although urinary symptoms should be investigated, the added value of dipstick testing is controversial. A Dutch study found no value of testing in such a population [Citation8]. The E.A.U. Guidelines has, based on expert views, a recommendation for dipstick testing to detect MiH in assessment of male L.U.T.S.
A recent study, which makes no distinction as to whether or not there were accompanying symptoms, demonstrated an overall 1.0% risk of malignancy in MiH patients under 60-years of age referred for investigation [Citation9]. In an accompanying editorial commentary, Lotan [Citation10] calculated that this figure was highly exaggerated, as most patients with this MiH never get referred.
At present, due to the lack of evidence, some national guidelines recommend against dipstick urine testing, as in Sweden [Citation11], while other national guidelines still recommend testing and even recommend repeat testing if the initial investigation is normal [Citation12]. Again, other guidelines recommend investigation after certain criteria are fulfilled, as for example in the U.K., where only older patients and those without a U.T.I. should be investigated [Citation13]. The E.A.U. guidelines do not give any specific recommendations on hematuria investigation.
Fast track pathways (F.T.P.) for patients with hematuria have recently been introduced in several countries, with the intention of improving the quality and timeliness of services. In Denmark, F.T.P. was introduced in 2008 and then 2015 in Sweden and Norway.
The authors of this project are all working within the Nordic urothelial cancer group that has the aim to increase cooperation in the management of this disease. As hematuria is the main presenting symptom in patients with bladder cancer we as an ad hoc network wanted to investigate possibilities to have a uniform view on investigation of this symptom. The specific aim of this paper is to describe the changes in the national recommendations regarding the assessment of MiH in the Nordic countries, especially in view of the introduction of F.T.P. and their impact on bladder cancer diagnosis and the use of health resources, by analysing data from the national cancer registries. Finally, based on these results, the aim would be to give arguments for a common Nordic guideline.
Materials and methods
Nordic
The national cancer registries in the Nordic countries, which have compulsory data entry, were the first of their kind in the world, and started in the 1940s and 1950s. They are all based on total populations, and each has its unique personal identity code system. The total population of these five countries now exceeds 26 million, and more than 100,000 new cancer cases are registered every year. In 2000, the registries had the N.O.R.D.C.A.N. programme developed, enabling easy access to basic cancer statistics, including incidence and mortality, in the Nordic countries on a regional level.
Sweden
The Swedish national guidelines from 2002 have stated that MiH should not be screened for and, if found incidentally, should not to be investigated, but have recommended the urgent investigation of patients with MaH without known cause. This recommendation was made more explicit in 2015 with the introduction of an F.T.P. when a lower age limit of 40 years was set for MaH. This was recently changed to 50 years based on the initial results showing very low cancer incidence in the 40–50 age group.
The Swedish national bladder register (S.N.R.U.B.C.) started in 1997 and is a nationwide quality register with coverage of 97% as compared to the Swedish Cancer Register. It includes detailed information on initial characteristics like stage and grade, but also initial management. This database is regularly updated with data from the Swedish Death register.
Denmark
In 1997, Danish national guidelines on bladder tumours recommended urological investigation in patients aged 50 or older if they had asymptomatic MiH [Citation14]. In later revisions this age limit was temporarily lowered to 40 years. When an F.T.P. was introduced in 2008, asymptomatic patients with MiH, less than 40 years old, were not recommended for investigation at all. However, all MaH patients and symptomatic MiH patients more than 40 years old were recommended for investigation: symptomatic was not defined by the type or severity of symptoms. Asymptomatic patients with MiH with age more than 40 years old were recommended for investigations, but outside F.T.P.
Based on two publications [Citation15,Citation16], Danish national guidelines were changed to effective from January 2016. Thereafter, asymptomatic patients with MiH were no longer to be investigated, irrespective of age, as the overall risk of malignancy was shown to be very low and did not exceed the expected incidence found by opportunistic screening in an age-matched cohort. In addition, the F.T.P. raised the age limit for investigating patients with symptomatic MiH in F.T.P. to 60 years, as the risk of malignancy below this age limit was found to be less than 5%. Throughout the period, all patients with MaH had been referred to the F.T.P. if no other obvious reason was found, irrespectively of age.
Detailed information on bladder cancer patients is available in the Danish Bladder Cancer Database that includes all patients diagnosed with bladder cancer in Denmark since 2012 [Citation17]. As opposed to the other countries, Ta-tumours are not considered as being cancer. Data in this database are automatically retrieved from the Danish Patient Registry and the Danish Central Pathology Registry with complete national coverage.
Norway
In Norway, the first national guideline for bladder cancer was established by the Norwegian Directorate of Health in 2013. The guideline was based on recommendations from the Norwegian Urological Cancer Group (N.U.C.G.) in 2005 which stated that MiH, in patients aged 50 years or more, should be referred for investigation to an urologist. Later the Norwegian urological association in their recommendations stated that asymptomatic MiH should be investigated after the age of 40, with exceptions for women with MiH that stopped after antibiotics for U.T.I., or after stone treatment.
The Norwegian F.T.P. for hematuria was established in 2015 and defined MiH as at least three positive dipsticks tests, with a 1 month gap between each test. MiH in smokers, or workers handling chemicals and older than 50 years, or in combination with pelvic pain were included in the pathway. A new revision of the Norwegian guidelines is under way and will suggest that asymptomatic MiH in patients without risk factors do not need investigation. For patients with symptoms or risk factors a certain age limit has not been set, but the suggestion is that it will be above 50 years. A national registry for bladder and urothelial cancer is being developed and will hopefully be finished in the next few years.
Finland
In Finland, there are no national guidelines on bladder cancer and the E.A.U. guideline is used instead, hence there is no clear instruction as to whether to investigate MiH.
One of the five Finnish University Hospital districts (Tampere) has given its official opinion that the investigation of asymptomatic MiH be abandoned, and this has been published in a national journal. In the other four academic districts there have not been official statements and there are differences in approaches. Many advocate risk-based approaches, i.e. investigations in older individuals and individuals with risk factors, such as smoking history or exposure to occupational chemicals. Nevertheless, there has been a clear trend towards less rigorous investigations. There is an ongoing discussion and it is likely that a national statement recommending no investigations in asymptomatic MiH will be made in the near future.
Iceland
In Iceland, there are no formal guidelines on bladder cancer or the work-up of hematuria except for local rules used, for instance, in the hospital emergency room. Consequently, doctors usually refer to the guidelines used in their training country when dealing with hematuria. This leads to diversity in diagnostic work-up of the patients involved, particularly when dealing with MiH where current international guidelines disagree substantially.
The different national approaches are detailed in and .
Table 1. Management of patients with hematuria in the different countries.
Table 2. Proportion (%) diagnosed with bladder cancer in fast track pathway.
Results
Nordic
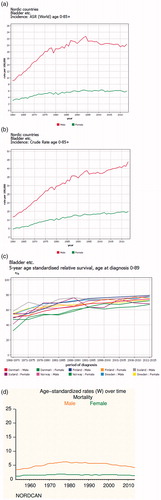
According to the combined Nordic cancer registries – N.O.R.D.C.A.N. – the age standardized incidence of urinary bladder cancer more than doubled, for both genders from 1960 until the 1990s, but after that has stabilized. The crude incidence on the other hand has continued to increase but at a somewhat lower rate. The increase is mainly in the oldest age category (Supplementary Figure 1). The incidence is markedly lower in Finland compared to other Nordic countries. In 2015, the age standardized rate per 100,000 inhabitants in Finland was 14.7 in males, compared to 19.5, 23.9, 24.8, and 22.3 in Sweden, Norway, Denmark, and Iceland, respectively. The corresponding rates for females were 3.5, 6.1, 7.8, 8.0, and 4.8. The male-to-female ratio is about 3:1 overall. The relative survival has increased in all countries, while the mortality has been stable. depict these data (Nordcan, http://www-dep.iarc.fr/N.O.R.D.C.A.N./).
Sweden
The crude incidence has increased 1.4-fold since 1997, while the age standardized incidence has remained stable. In a report from S.N.R.U.B.C., covering the period up to 2011, the proportion of muscle-invasive tumours decreased despite abandoning MiH testing early in this period [Citation18]. The relative survival showed no statistically significant change over this time, but in a recent update the relative and observed survival improved significantly after 2012, and this was similar at all disease stages [Citation19]. The corresponding mortality rate during this period was stable at about seven per 100,000 inhabitants.
In the Swedish F.T.P., 6080 patients were referred from primary care during the first year. Of these, 76% were accepted by urology services for investigation, and 15% of these were found to have bladder cancer. Thus, about half of the patients with bladder cancers yearly were diagnosed in this pathway and the rest outside the pathway. The time from referal to diagnosis has been measured since the start of the S.N.R.U.B.C., as one of five quality indicators. The median time was stable at 35 days until the introduction of the F.T.P., following which the time was reduced by 10 days.
Denmark
In the period 2013–2015, the number of F.T.P. investigations because of hematuria was approximately 13,000 per year, with 17.5% of patients found to have invasive urinary tract cancer. This includes renal cancer, invasive upper tract urothelial tumours, urethral cancer and invasive bladder cancer, whereas patients with findings of non-invasive Ta-tumour were not included in this percentage. Following the increase in the age limit for investigating symptomatic MiH from 40–60 years of age, the total number of hematuria referrals to the F.T.P. was only slightly reduced to ∼ 12,000 per year in the following years, 2016 and 2017. The number of positive findings increased slightly in the same period, leading to positive findings in 21.8%. Still almost half of the registred new cancers are diagnosed outside of the F.T.P.
The total number of newly-diagnosed patients with bladder tumour did not change significantly following the change to more restrictive criteria.The percentage of muscle-invasive bladder cancer did not change significantly either when comparing the period before change in guidelines to the period after (24% vs 25%). However, a significant change in non-invasive Ta vs T1-tumours was noted. Thus, the percentage of patients with Ta tumours was reduced from 54% of all newly-diagnosed patients in the first period to 48% in the latest period. Conversely, T1 tumours which constituted 22% in the first period, increased to 26% in the latest period. This change in stage of NMIBC tumours was significant (p < 0.05) and may indicate a worse prognosis at diagnosis following change in national guidelines, due to omitting the investigation in patients with asymptomatic MiH.
Norway
The incidence of bladder cancer has been steadily rising in Norway. The introduction of the F.T.P. does not seem to have changed this tendency substantially. It is not possible to discriminate patients with MiH and MaH. In 2017, 5275 patients were included, of which 33% where registered as having cancer. This constituted 74% of all newly-diagnosed bladder cancer patients and 90% of these patients, diagnosed with bladder cancer, were diagnosed within 32 days of referral, which is the F.T.P. standard.
Discussion
Views on the assessment of patients with hematuria and especially MiH, varies in the Nordic countries as in the rest of the world. The main reason is lack of high level evidence regarding optimal investigation practices. We believe that our unique nationwide population based bladder cancer registry data could be highly informative in this regard, especially with the gradual introduction of F.T.P.s and its effect on more standardization of the investigation.
In the Nordic countries, ∼ 6000 patients are diagnosed annually with bladder cancer. The crude incidence has increased by 50% in the last two decades, making it an important national health challenge. The two most probable causes are an increasing population and a changing age structure, with a higher number of elderly. While survival has increased, the most important measure of progress at a population level, mortality has not improved as it has in other cancers, as for example in breast cancer.
Bladder cancer is, to a large extent, a preventable disease and the main aetiological factor, smoking, has drastically reduced due to public rules and regulations. But until these and other primary prevention measures have an effect on the whole population, secondary prevention or improved early detection remains very important in reducing mortality. One measure could be to screen at risk populations. As pointed out above this has not proved to be successful, but this could be due to a lack of effective screening methods. Consequently, an effective approach to hematuria, the most common presenting symptom of bladder cancer, seems most appropriate.
There is no controversy that MaH should be rapidly investigated, and further support for this comes from our national F.T.P.s with the finding of cancer in almost 20% of referred patients. What is more controversial is what are the sub-groups that can be excluded? There is an ongoing national discussion in Sweden about the necessity to investigate all patients with U.T.I. In the recent recommendations, an age limit of 50 years was set to decrease investigation of, for example, younger women who have a higher risk of urinary tract infections. Denmark has excluded this category from diagnostic work up to some extent.
Diagnostic work up of MiH is more controversial due to lack of evidence. For almost 20 years, Sweden has not recommended investigation of all types of MiH, and the same policy has recently been adopted in the Danish F.T.P. The other Nordic countries are moving towards a similar conclusion in the absence of accompanying symptoms, such as L.U.T.S. and bladder pain.
One crucial question is whether or not the exclusion of MiH investigation leads to a delayed diagnosis and consequently more advanced tumours. An American study found that MiH at the time of diagnosis was associated with lower disease stage as compared to those with MaH, and suggested that a delayed diagnosis could influence survival [Citation20]. However the authors acknowledged the evidence limitation of this retrospective observational cohort study. In Denmark, the increase in more high risk non-muscle invasive (N.M.I.B.C.) tumours, after abandoning MiH inclusion in the F.T.P., is potentially worring and needs to be closely watched. In Sweden, no such changes in tumour characteristics has been seen. Although the investigation of MiH may allow earlier diagnosis, in our public health systems the cost of this must be put in perspective with other health expenditures. In Denmark, a cut-off of less than 5% cancer detection rate was used to remove low-risk populations from the F.T.P., and seems to be an acceptable compromise.
The introduction of F.T.P.s, while successful, has incurred an extra cost on the healthcare systems. Denmark has experienced a significant increase in the number of patients referred with both symptomatic and asymptomatic MiH, despite the latter not being in the F.T.P. inclusion criteria. Also, in Sweden the number of investigations has doubled due to the F.T.P. introduction, despite stringent criteria. This has led to recent changes in the inclusion criteria; in Denmark investigating asymptomatic MiH has been stopped, and the age limit for investigating symptomatic MiH has been raised, and in Sweden the age limit for MaH investigation has been raised.
Based on reviewing the literature and our common experience, the authors recommend that asymptomatic MiH per se should be abandoned as indication for cystoscopy and radiological investigation. The association between MiH and symptoms and the importance of symptoms in making the diagnosis of bladder cancer in patients with MiH is uncertain and data selection bias may be a problem. Therefore, at present symptomatic MiH can be investigated according to guidelines, for symptomatic and patients with higher risk factors for urothelial cancer, but not necessarily be included in the fast track pathway. It is a priority to establish these relationships so that only those patients who are likely to benefit from investigation are included in F.T.P.s. This is not only essential in ensuring that F.T.P.s are cost-effective, but also in ensuring that the psychological morbidity for the many patients with MiH and a very low chance of being diagnosed with malignancy is eliminated by not including them in the F.T.P.
A limitation of our analysis is the lack of knowledge of the cost-effectiveness associated with introduction of F.T.P.s. The impact on reduction in investigation time has been considerable but whether this translates into a reduced mortality must be established. As shown above almost half of the newly-diagnosed cancers are not in the F.T.P. pathways. Probably this proportion will decrease with time and then a more comprehensive analysis can be performed on the impact on healthcare. We have no complete quality-of-life data for the Nordic region but, in the Patient Reported Experience Measures in Sweden, 86% answered that they were satisfied having been included in a F.T.P. on suspicion of bladder cancer. Interestingly, a few commented that the rapid investigation increased their anxiety.
Conclusion
Based on literature and data from the Nordic region we recommend abandoning asymptomatic MiH as an indication for investigation. The value of MiH as part of the investigation of patients with urological symptoms or certain risk factors is unclear, but continued analysis of our registries hopefully should give us more information in the near future.
On the other hand, MaH is a serious symptom which necessitates urgent and complete investigations. The introduction of F.T.P.s has increased knowledge on the epidemiology of this patient category and seems to fulfil the intended strategy to make the investigation more rapid. It remains to be seen whether the same applies to sub-groups of MiH patients.
Supplementary figure. Incidence trends per 100,000 inhabitants in different age groups for males and females in the Nordic countries
Supplemental Material
Download MS Power Point (430.7 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Jones R, Latinovic R, Charlton J, et al. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. Bmj. 2007;334:1040–1048.
- Carel RS, Silverberg DS, Kaminsky R, et al. Routine urinalysis (dipstick) findings in mass screening of healthy adults . Clin Chem. 1987;33:2106–2108.
- Schroeder GL, Lorenzo-Gomez MF, Hautmann SH, et al. A side by side comparison of cytology and biomarkers for bladder cancer detection. J Urol. 2004;172:1123–1126.
- Bangma CH, Loeb S, Busstra M, et al. Outcomes of a bladder cancer screening program using home hematuria testing and molecular markers. Eur Urol. 2013;64:41–47.
- Steiner H, Bergmeister M, Verdorfer I, et al. Early results of bladder-cancer screening in a high-risk population of heavy smokers. BJU Int. 2008;102:291–296.
- Pesch B, Nasterlack M, Eberle F, et al. The role of haematuria in bladder cancer screening among men with former occupational exposure to aromatic amines. BJU Int. 2011;108:546–552.
- Krogsbøll LT, Juhl Jørgensen K, Gøtzsche PC. Screening with urinary dipsticks for reducing morbidity and mortality. First published: 27 January 2015 Cochrane DOI: 10.1002/14651858.CD010007.pub2
- Ezz el Din K, Koch WF, de Wildt MJ, et al. The predictive value of microscopic haematuria in patients with lower urinary tract symptoms and benign prostatic hyperplasia. Eur Urol. 1996;30:409–413.
- Tan WS, Feber A, Sarpong R, et al. Who Should Be Investigated for Haematuria? Results of a Contemporary Prospective Observational Study of 3556 Patients. Eur Urol. 2018;74:10–14.
- Lotan Y, Editioral comment to Tan, et al. Re: Who Should be Investigated for Hematuria? Results of a Contemporary Prospective Observational Study of 3556 Patients. Eur Urol. 2018;74:15–16.
- Malmström PU. Time to abandon testing for microscopic haematuria in adults?. Bmj. 2003;326:813–815.
- Davis R, Jones JS, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188:2473–2481.
- Jefferies ER, Brewster SF. BAUS Section on Oncology. Urological recommendations from the National Institute for Health and Care Excellence (NICE) Guideline, June 2015: Suspected cancer: recognition and referral. BJU Int. 2016;117:857–860.
- Ugeskr Laeger. Urinary bladder tumors. Report of the Danish Bladder-cancer Committee 1993. 1997;159:1–14.
- Ordell Sundelin M, Jensen JB. Asymptomatic microscopic hematuria as a predictor of neoplasia in the urinary tract. Scand J Urol. 2017;51:373–375.
- Elmussareh M, Young M, Ordell Sundelin M, et al. Outcomes of haematuria referrals: two-year data from a single large university hospital in Denmark. Scand J Urol. 2017;51:282–289.
- Hansen E, Larsson H, Nørgaard M, et al. The Danish Bladder Cancer Database. Clin Epidemiol. 2016;8:439–443.
- Jahnson S, Hosseini Aliabad A, Holmäng S, et al. Swedish National Registry of Urinary Bladder Cancer: No difference in relative survival over time despite more aggressive treatment. Scand J Urol. 2016;50:14–20.
- Malmström PU, Liedberg F, Sherif A, et al. Is there finally an increasing survival of patients with urinary bladder cancer? A nationwide study in Sweden 1997–2016. European Urology Supplements 2018;17:858.
- Ramirez D, Gupta A, Canter D, et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int. 2016;117:783–786.