Abstract
Purpose: All types of surgery are associated with complications. The debate is ongoing whether robot-assisted radical prostatectomy can lower this risk compared to open surgery. The objective of the present study was to evaluate post-operative adverse events leading to readmissions, using clinical records to classify these adverse events systematically.
Materials and methods: A prospective controlled trial of men who underwent robot-assisted laparoscopic (RALP) or retropubic radical prostatectomy (RRP) at 14 departments of Urology (LAPPRO) between 2008 and 2011. Data on all readmissions within 3 months of surgery were collected from the Patient registry, Swedish Board of Health and Welfare. For each readmission the highest Clavien-Dindo grade was listed.
Results: A total of 4003 patients were included in the LAPPRO trial and, after applying exclusion criteria, 3706 patients remained for analyses. The results showed no statistically significant difference in the overall readmission rates (8.1 vs. 7.1%) or readmission due to major complications (Clavien-Dindo ≥3b, 1.7 vs. 1.9%) between RALP and RRP within 90 days after surgery. Patients subjected to lymph-node dissection (LND) had twice the risk for readmission as men not undergoing LND, irrespective RALP or RRP technique. Blood transfusion was significantly more frequent during and within 30 days of RRP surgery (16 vs. 4%). Abdominal symptoms were more common after RALP.
Conclusions: There is a substantial risk for hospital readmission after prostate-cancer surgery, regardless of technique; although major complications are rare. Regardless of surgical technique, attention should be focused on specific types of complications.
Introduction
Robot-assisted laparoscopic radical prostatectomy (RALP) was introduced and widely implemented without high-level evidence of its superiority as compared to the retropubic radical prostatectomy (RRP). A debate continues regarding the short- and long-term pros and cons of RALP [Citation1–3]. In the past, surgical reports only listed complications of surgical procedures that occurred during the index hospital stay and only listed complications directly related to the surgical procedure. We are now more aware that post-operative complications can occur later; reports of complications within 90 days are now common, including all adverse events as post-operative complications. The classification system introduced by Clavien and Dindo further improved the quality and comparability of reporting complications and has been rapidly adopted by the surgical community [Citation4].
In LAPPRO (Laparoscopic Prostatectomy Robot Open), a prospective comparative trial of two operative techniques of radical prostatectomy, we have earlier presented patient-reported adverse events within 3 months after the operation. Using that data it was not possible to classify the events using the Clavien-Dindo system. In order to explore serious post-operative adverse events leading to readmission, we used medical records to classify these adverse events systematically.
Method
This study took place within LAPPRO (Laparoscopic Prostatectomy Robot Open); a prospective comparative trial between RALP and RRP [Citation5] using data from 14 Swedish Urology departments in the years of 2008–2011 [Citation6]. Seven centres used the robot-assisted approach and seven centres used the open approach. The inclusion criteria were age <75 years, the ability to read and write Swedish, informed consent, tumour stage cT1, cT2, or cT3 (TNM Classification of Malignant Tumors) [Citation7] with no signs of distant metastases, and a prostate-specific antigen level of <20 ng/mL. Primary outcome was post-operative adverse events leading to readmissions up to 3 months after surgery. Data on all readmissions within 3 months of surgery were collected from the Patient registry, Swedish Board of Health and Welfare. These data made it possible to collect original documentation for all readmissions. For each readmission only the highest Clavien-Dindo grade was listed, even if several complications had occurred (). A quality control of grading was made by external reviewers. Data on blood transfusions within 30 days of surgery were retrieved from the Departments of Transfusion medicine of the participating hospitals. Hence, transfusions given during index admission were included. Causes for readmission defined by ICD-10 codes were grouped into seven groups, presented in (). The Regional Ethical Review Board in Gothenburg (No 277-07) approved the study. The trial is registered in the Current Controlled Trials database (ISRCTN06393679).
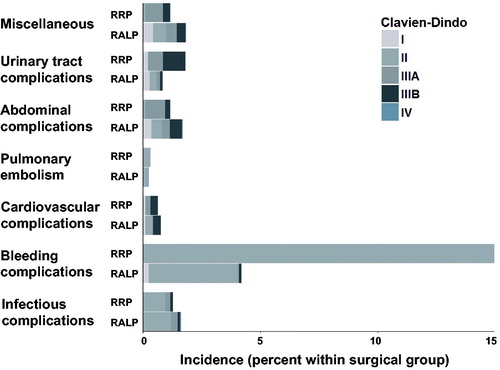
Figure 1. Comparison between open and robot-assisted surgery concerning grade of Clavien-Dindo complications.
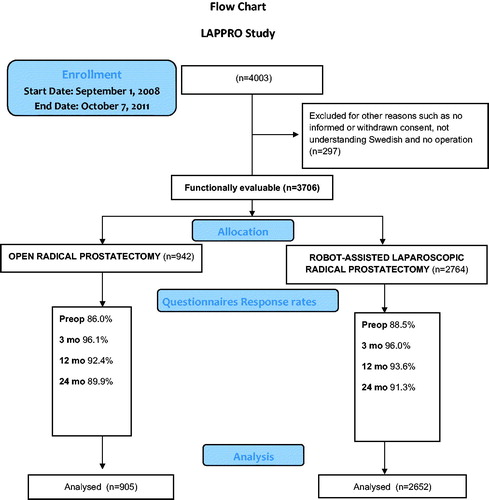
Table 1. Clavien-Dindo classification.
Table 2. Causes for readmission defined by ICD-10 codes.
Statistics
The pre-defined statistical analysis plan can be found in the Supplementary Appendix. The percentage of patients with one or more re-admissions within 3 months was analyzed with the modified Poisson regression approach of Zou [Citation8]. The number of readmissions was analyzed with negative binomial regression. Covariates used for adjustments concerning readmissions were those used in the previously published analysis of patient-reported complications with the addition of type of residence (urban/rural) [Citation9]. For the two secondary variables, the percentage of patients with Clavien-Dindo classifications 3b or higher and the percentage of patients given a transfusion, the modified Poisson regression was used. Age at surgery, prostate weight, and residence were used as covariates in the statistical model. Results are presented as ratios (RALP vs. RRP), 95% confidence intervals, and p-values. No correction for multiplicity was performed. Multiple imputation by chained equations was used to handle missing values in the different covariates used for adjustment in the analyses [Citation10]. Statistical analyses were performed using SAS vs. 9.4 (SAS Institute Inc., Cary, NC).
Results
In total 4003 patients were included in the LAPPRO trial. Applying exclusion criteria, 3706 patients remained for analyses, where 2764 men were operated on by RALP and 942 by RRP (). Men operated on by the robot-assisted procedure had higher clinical tumour stages, but lower pre-operative PSA levels. Limited lymph node dissection was significantly more common during open procedures, whereas extended lymph node dissection was more common in the robot-assisted group ( and ).
Table 3. Baseline characteristics of the patientsTable Footnotea.
Table 4. Post-operative tumour characteristics and prostate weightTable Footnotea.
There was no statistically significant difference in readmission rates or in major complications defined as Clavien-Dindo 3b or higher between the surgical techniques, RR = 1.16 (95% C.I. = 0.88–1.53) and RR = 0.91 (95% C.I. = 0.52–1.58), respectively (). Furthermore, there was no statistically significant difference in men operated on by experienced surgeons, defined as having performed 100 operations or more, or the sub-groups of men who underwent lymph-node dissection (LND), regarding readmission rates and major complications. However, in a sub-group analysis patients undergoing LND, regardless of operative technique, had a statistically significant higher risk for readmission according to the adjusted analysis (14 vs. 7%, p-value <0.001).
Table 5. Comparison between open and robot-assisted surgery.
Patients in the RRP group received significantly more blood transfusions than patients in the RALP group (4 vs. 16%, RR 0.25 (95% C.I. = 0.20–0.33)) during surgery and until 30 days after surgery (). RALP was more often associated with grade 1 complications, which corresponds to any deviation from the normal post-operative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions, while RRP was more often associated with grade 2 complications, which corresponds to, for example, blood transfusions and antibiotics (). In the RALP group, readmissions due to abdominal symptoms were significantly more common, while disorders of lymphatic vessels and lymph nodes leading to readmission were significantly more common in the RRP group (). There was no statistically significant difference in rates of pulmonary embolism between the surgical groups.
Table 6. Comparison between open and robot-assisted surgery concerning complications.
Table 7. Frequency table over complications causing readmissions after RRP vs. RALP
Discussion
In this prospective comparative trial based on inpatient registry data we found no statistically significant difference in re-admittance rates between RRP and RALP or the rate of major complications defined as Clavien-Dindo 3b or higher. These results are consistent with our previously published paper based on patient reports [Citation11]. However, the reasons for re-admittance differed between the two techniques. In the robotic group, abdominal symptoms were more common, while blood transfusions were more common in the RRP group.
Leow et al. [Citation12] could, in a registry based study based on inpatient data from 629,593 patients operated on in 449 hospitals in the US between 2003 and 2013, show no difference in major complications (Clavien-Dindo ≥3) between RRP and RALP In that study minor complications not requiring intervention (Clavien-Dindo <3) were slightly more common and, in the RRP group and also in correlation with our material, the number of patients receiving a blood transfusion were higher in the RRP group. Similarly, the randomized controlled trial by Yaxley et al. [Citation13] could not find any statistically significant difference between the two surgical approaches with 308 assessable patients.
Transfusion rates of 16% in RRP cases were higher than in the reports by Trinh et al. [Citation14] from a nationwide inpatient sample from high volume centres in the U.S.A. reporting 8% of blood transfusions. However, Alemozaffar et al. [Citation15] reported transfusion rates as high as 30% in RRP cases from the Health Professionals Follow-up Study. The present study included both high and low volume centres, which could in part explain the relatively high rate of transfusions. Additionally, differences between centres might be explained by arbitrary and person-dependent transfusion limits. Blood transfusion is a grade 2 complication according to the Clavien-Dindo classification and as such is often downplayed in the academic literature, as a minor adverse event. For prostate cancer patients during or after radical prostatectomy, the effect of transfusion remains a matter of debate. It is debated whether transfusion could be a negative prognostic factor, leading to earlier cancer recurrence or shorter overall survival [Citation16–23]. However, the evidence is still conflicting regarding this matter. It is postulated that transfusion has an immunosuppressing action, leading to less tolerance in infections and cancerous development. Another argument is related to the increased intraoperative blood loss, which obscures the operative field, causing a less accurate excision of the tumour and positive surgical margins. In that sense, our study concurs with the available literature that RALP is advantageous compared to RRP, in terms of blood transfusion, which is expected since RALP is a minimally invasive procedure.
In line with our previous patient reported results, lymph-node dissection generally increased the risk of complications for both RRP and RALP [Citation11]. Keskin et al. [Citation24] could, in a study of 521 consecutive patients undergoing RALP and LND, report that 9% of all patients developed a lymphocele detectable by ultrasonography and 2.5% developed symptomatic lymphoceles. In another study of 79 consecutive patients followed after RALP and LND, Orvieto et al. reported that as many as 51% developed a lymphocele detectable on ultrasonography, and over 15% developed clinical symptoms [Citation25]. A substantial percentage of lymphoceles require intervention, in most cases percutaneous drainage in local anesthetics and, thus, having a Clavien-Dindo classification of 3a. In our study, virtually all lymph node dissections in the RALP group were extended, while most in the RRP group were limited. It is likely that more extensive dissection of lymph nodes carries a higher likelihood of post-operative lymphocele and, thus, results in a higher rate of intervention classified as Clavien-Dindo 3a. The trans-abdominal approach of the RALP procedure will also presumably lead to lymph leaking into the abdominal cavity and, thereby, cause more symptoms than extra-peritoneal leakage would.
In contrast to our finding using patients’ reports, where we found a higher risk of thromboembolic complications after RRP [Citation11], we found no statistically significant difference in occurrence of pulmonary embolism leading to hospital admittance between the surgical groups. Furthermore, there were no readmittances due to deep venous thrombosis (DVT.) alone in this material. A likely explanation of this difference in outcome is that, in Sweden, patients with DVT would mainly be treated as outpatients. Two large studies in the early 2000s by Lapidus et al. [Citation26] and Bäckman et al. [Citation27] could establish that outpatient treatment of DVT was both safe and cost-effective. The consequence is that today more than 80% of all patients with DVT are treated as outpatients in Sweden and, as patients undergoing radical prostatectomy should have a long life expectancy to benefit from the procedure, it is likely that they are more fit than the general population developing DVT and thereby have a higher rate of outpatient treatment. However, a previous report by Van Hemelrijck et al. [Citation28] evaluating a large population-based cohort, using data from the Prostate Cancer Database Sweden (PCBaSe), showed that surgery for prostate cancer was associated with hospitalization for thromboembolic diseases.
In our previous published paper based on patient-reported outcomes, we reported that RALP and RRP had comparable and not statistically significant different rates of 90-day readmissions (9.3% vs. 7.7%) [Citation9]. These numbers are comparable to a study from the population based, nationwide PCBaSe (Prostate Cancer data Base Sweden) reporting 9% vs. 10% 90-day readmission rates after robot-assisted and retropubic radical prostatectomy, respectively [Citation29]. In this study, we studied the same outcome using healthcare reported outcomes this time, as generated by The National Inpatient Registry. Contrary to that notion, in the SPCG-7 randomized trial comparing radiation to hormonal deprivation, Steinsvik et al. [Citation30] have reported that physicians may over-report complications compared to the actual suffering patients. On the other hand, Litwin et al. [Citation31] have suggested that patients reported more complications, as seen in the CaPSURE study. In our study, there was agreement between patients and healthcare professionals. This could be attributed to LAPPROs designs and strengths. We could hypothesize that patient-reported outcomes might offer a high-quality dataset, from which important and accurate conclusions could be drawn, when care is taken for data gathering standardized reporting.
The strengths in our study include the prospective controlled design, size, short inclusion period, high response rate, use of validated measures and the neutral third-party approach [Citation32]. We made sure to obtain accurate information of known and suspected risk factors for adjustment. The systematic classification of complications using a widely adopted instrument revealed important differences between surgical techniques concerning the post-operative course in comparison with patient-reported data. One limitation in the original dataset was that readmissions as reported by healthcare professionals in C.R.F.s probably were limited to that particular hospital. A certain proportion of the participants were operated on not at the neighboring hospital, but referred to a more distant one. Thus, readmissions could have been missed. To counteract this possible limitation that could theoretically have been skewed regarding type of surgical technique, we choose to retrieve information from the National Inpatient Registry, where all hospitals by law must report data on all inpatient care episodes.
Conclusion
There is a substantial risk for re-admittance after radical prostatectomy, regardless of surgical technique. Complications are in most cases of low-to-moderate severity. Severe complications are rare for both open and robotic prostatectomy, but differ in types.
Disclosure statement
The authors report no conflicts of interest.
References
- Tewari A, Sooriakumaran P, Bloch DA, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15.
- Ficarra V, Minervini A, Antonelli A, et al. A multicentre matched-pair analysis comparing robot-assisted versus open partial nephrectomy. BJU Int. 2014;113:936–941.
- De Carlo F, Celestino F, Verri C, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: surgical, oncological, and functional outcomes: a systematic review. Urol Int. 2014;93:373–383.
- Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 Open Retropubic Radical Prostatectomies at the Karolinska University Hospital, Sweden. Urology 2009; S0090–4295:02762–02769.
- Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68:216–225.
- Thorsteinsdottir T, Stranne J, Carlsson S, et al. LAPPRO: a prospective multicentre comparative study of robot-assisted laparoscopic and retropubic radical prostatectomy for prostate cancer. Scand J Urol Nephrol. 2011;45:102–112.
- Sobin LH, Compton CC. T.N.M. seventh edition: what's new, what's changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 2010;116:5336–5339.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706.
- Wallerstedt A, Tyritzis SI, Thorsteinsdottir T, et al. Short-term results after robot-assisted laparoscopic radical prostatectomy compared to open radical prostatectomy. Eur Urol. 2015;67:660–670.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399.
- Wallerstedt A, Tyritzis SI, Thorsteinsdottir T, et al. Short-term results after robot-assisted laparoscopic radical prostatectomy compared to open radical prostatectomy. Eur Urol. 2015;67:660–670.
- Leow JJ, Chang SL, Meyer CP, et al. Robot-assisted versus open radical prostatectomy: A contemporary analysis of an all-payer discharge database. Eur Urol. 2016;70:837–845.
- Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 2016;388:1057–1066.
- Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol. 2012;61:679–685.
- Alemozaffar M, Sanda M, Yecies D, et al. Benchmarks for operative outcomes of robotic and open radical prostatectomy: results from the Health Professionals Follow-up Study. Eur Urol. 2015;67:432–438.
- Kim JK, Kim HS, Park J, et al. Perioperative blood transfusion as a significant predictor of biochemical recurrence and survival after radical prostatectomy in patients with prostate cancer. PLoS One. 2016;11:e0154918.
- Lloyd JC, Banez LL, Aronson WJ, et al. Estimated blood loss as a predictor of PSA recurrence after radical prostatectomy: results from the SEARCH database. BJU Int. 2010;105:347–351.
- Oefelein MG, Colangelo LA, Rademaker AW, et al. Intraoperative blood loss and prognosis in prostate cancer patients undergoing radical retropubic prostatectomy. J Urol. 1995;154:442–447.
- Paul R, Schmid R, Busch R, et al. Influence of blood transfusions during radical retropubic prostatectomy on disease outcome. Urology 2006;67:137–141.
- Ford BS, Sharma S, Rezaishiraz H, et al. Effect of perioperative blood transfusion on prostate cancer recurrence. Urol Oncol. 2008;26:364–367.
- Gallina A, Briganti A, Chun FK, et al. Effect of autologous blood transfusion on the rate of biochemical recurrence after radical prostatectomy. BJU Int. 2007;100:1249–1253.
- Ness PM, Walsh PC, Zahurak M, et al. Prostate cancer recurrence in radical surgery patients receiving autologous or homologous blood. Transfusion 1992;32:31–36.
- Boehm K, Beyer B, Tennstedt P, et al. No impact of blood transfusion on oncological outcome after radical prostatectomy in patients with prostate cancer. World J Urol. 2015;33:801–806.
- Keskin MS, Argun OB, Obek C, et al. The incidence and sequela of lymphocele formation after robot-assisted extended pelvic lymph node dissection. BJU Int. 2016;118:127–131.
- Orvieto MA, Coelho RF, Chauhan S, et al. Incidence of lymphoceles after robot-assisted pelvic lymph node dissection. BJU International. 2011;108:1185–1190.
- Lapidus L, Borretzen J, Fahlen M, et al. Home treatment of deep vein thrombosis. An out-patient treatment model with once-daily injection of low-molecular-weight heparin (tinzaparin) in 555 patients. Pathophysiol Haemo T. 2002;32:59–66.
- Backman K, Carlsson P, Kentson M, et al. Deep venous thrombosis: a new task for primary health care. A randomised economic study of outpatient and inpatient treatment. Scand J Prim Health Care. 2004;22:44–49.
- Van Hemelrijck M, Garmo H, Holmberg L, et al. Thromboembolic events following surgery for prostate cancer. Eur Urol. 2013;63:354–363.
- Friethriksson JO, Holmberg E, Adolfsson J, et al. Rehospitalization after radical prostatectomy in a nationwide, population based study. J Urol. 2014;192:112–119.
- Steinsvik EA, Fossa SD, Axcrona K, et al. Do perceptions of adverse events differ between patients and physicians? Findings from a randomized, controlled trial of radical treatment for prostate cancer. J Urol. 2010;184:525–531.
- Litwin MS, Lubeck DP, Henning JM, et al. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: results of the CaPSURE database. J Urol. 1998;159:1988–1992.
- Mansson A, Henningsohn L, Steineck G, et al. Neutral third party versus treating institution for evaluating quality of life after radical cystectomy. Eur Urol. 2004;46:195–199.