Abstract
Background: Response to neoadjuvant cisplatin treatment in bladder cancer has been linked to expression of Bcl-2 protein by cancer cells. The objective of this study was to test Bcl-2 as a predictive marker of neoadjuvant cisplatin chemotherapy response in a patient cohort from randomized cystectomy trials.
Methods: Tumor samples were taken from 247 patients with T2–T4 bladder cancer enrolled in two randomized trials comparing cystectomy with or without neoadjuvant chemotherapy. Tissue microarrays from pre-intervention transurethral resection specimens were assessed for Bcl-2 protein status by immunohistochemistry. Extension of staining above 10% was regarded as positive. Downstaging and survival ratios in relation to Bcl-2 immunoreactivity and neoadjuvant chemotherapy utilization were calculated using the log rank test and multivariate Cox proportional hazards regression analyses.
Results: Bcl-2 expression was positive in 38% and negative in 62% of the 236 evaluable patients. Bcl-2 negative patients receiving neoadjuvant chemotherapy had a significant increase in survival (p = 0.009), while Bcl-2 positive patients showed no difference (p = 0.4). However, the interaction variable between neoadjuvant chemotherapy and biomarker status was not significant (p = 0.38). When the prognostic value was assessed in the no-chemotherapy group, 5-year overall survival times were significantly better among Bcl-2 positive patients than among Bcl-2 negative patients (42 months vs 33 months, p = 0.04), but again Bcl-2 status did not remain independent when other factors were adjusted. Also, in a multivariate analysis with all patients, Bcl-2 was not significant.
Conclusions: Bcl-2 status is not an independent predictor of neoadjuvant cisplatin chemotherapy response and is not prognostic in muscle-invasive bladder cancer.
Introduction
Radical cystectomy is the standard treatment in muscle-invasive urothelial bladder cancer (M.I.B.C.). Unfortunately, this treatment provides a 5-year survival rate of only ∼50% [Citation1]. Neoadjuvant cisplatin-containing combination chemotherapy has been shown to improve overall survival (O.S.) by ∼5–8% at 5 years [Citation2,Citation3], and is recommended for patients with localized (T2–T4a) muscle-invasive bladder cancer and adequate renal function [Citation4]. However, the proportion of patients given neoadjuvant treatment is low. The major reasons for this are that the survival benefit is low when given to all patients, and patients not responding to neoadjuvant chemotherapy would be exposed to unnecessary treatment-related toxicities [Citation3,Citation5]. There is an urgent need for markers suitable for predicting the outcome of this treatment.
Apoptosis is the programmed cell death process which maintains a healthy balance between cell survival and cell death in the organism. It can be initiated by both intrinsic and extrinsic signals [Citation6]. One of the basic biological mechanisms behind cancer chemotherapy is to drive tumor cells into apoptotic cascade. The mode of cisplatin action has been linked to its ability to crosslink with the purine bases on D.N.A., thus interfering with D.N.A. repair mechanisms, causing D.N.A. damage, and subsequently inducing apoptosis in cancer cells [Citation7]. The mechanism by which damage recognition results in apoptosis is unclear; however, the induction of p53 following D.N.A. strand breaks leads to apoptosis via the intrinsic pathway and contributes to the cytotoxicity of cisplatin [Citation8]. When proteins involved in the apoptosis process become non-functional, the result may be increased drug resistance and cancer progression.
Bcl-2 family proteins are central regulators of this intrinsic pathway [Citation9]. Some of the members of this family (e.g. BAX and BAK) are pro-apoptotic, while others (e.g. Bcl-2 and Bcl-XL) are anti-apoptotic [Citation10]. The development of apoptosis depends on the balance between pro-apoptotic and anti-apoptotic proteins, and these members are involved in the downstream action by cisplatin [Citation8]. Bcl-2 protein is located at the intracellular membranes such as the mitochondrial membrane, nuclear membrane, and endoplasmic reticulum, and so may act to control ion channels, modulating caspase activation or inhibiting cytochrome c export [Citation11]. It also represents transcriptional targets for the p53 tumor suppressor protein that induces termination of the cell cycle or apoptosis as a reduction to D.N.A. damage.
Immunohistochemical Bcl-2 expression has been studied for its prognostic value and predictive role for patients receiving chemotherapy for various cancer types, with conflicting results [Citation12–14]. Similarly, conflicting results have been reported for urothelial cancer. Bcl-2 has been suggested to correlate with response to neoadjuvant chemotherapy before cystectomy.
In a previous study, our group showed that emmprin could be a potential marker predicting neoadjuvant cisplatin response in cystectomy patients [Citation15]. In that study, the patient material and dataset used were based on two randomized trials comparing cystectomy with or without neoadjuvant cisplatin. Having a control arm without chemotherapy made it possible to separate the predictive and prognostic values of the marker studied. In this study, using the same cohort of patients, we aimed to validate the predictive value of Bcl-2 regarding neoadjuvant chemotherapy response that was recently reported in a study by Kiss et al. [Citation16]. Our pre-specified hypothesis was that bladder cancer patients with negative Bcl-2 expression in tumor tissue would have an improved response to neoadjuvant chemotherapy compared to patients with Bcl-2 positive tumors.
Methods
Patient datasets
This study used the patient datasets from two prospective randomized trials by the Nordic collaborative group [Citation2] comparing cystectomy with vs without neoadjuvant chemotherapy. The total dataset was composed of 642 patients with primary T1G3 or T2–T4aNXM0 urothelial bladder cancer who were operated on between 1985 and 1997. Briefly, in the first trial, Nordic collaborative study 1 (N.C.S. 1), the intended chemotherapy was two cycles of cisplatin 70 mg/m2 intravenously and doxorubicin 30 mg/m2 intravenously. All patients in both the neoadjuvant and the control arm were planned to receive irradiation preoperatively, consisting of 4 Gy daily for 5 consecutive days. In the second trial (N.C.S. 2), three cycles of cisplatin 100 mg/m2 intravenously and methotrexate 250 mg/m2 intravenously were planned. Cystectomy was performed with lymph node dissection of the obturator fossa. The selection of 250 patients for this study is explained in detail elsewhere [Citation15] (see also Supplementary Figure 1). Today, neoadjuvant chemotherapy is not routinely applied for T1G3 tumor disease going into radical cystectomy. To prevent the possible influence on survival analyses, T1G3 patients (n = 3) were excluded from these datasets, leaving 247 patients with T2–T4aNXM0 disease for inclusion. All the materials were collected with ethical committee approval (approval no. 2008: 136).
Bcl-2 staining
Tumor specimens obtained during pre-treatment transurethral resection (T.U.R.) were preserved as formalin-fixed paraffin-embedded (F.F.P.E.) tissues. Representative tissue areas from F.F.P.E. materials were identified by a pathologist (C.B.), and two tissue microarrays (T.M.A.s) consisting of 1 mm cores were obtained from these areas. The T.M.A.s were constructed with an automated instrument (ATA-27, Beecher Instruments, Sun Prairie, W.I.). Immunohistochemistry (I.H.C.) was performed as previously reported using an Autostainer 480 instrument (Lab Vision, Fremont, C.A.) [Citation17]. Antibody type and concentrations were: DAKO, clone BCL-2 124, dilution 1:100. The I.H.C. method had previously been tested and evaluated by the core facility of SciLifeLab, Uppsala University producing the Human Protein Atlas [Citation18]. Negative controls were produced using Universal Negative Control Serum (A. Menarini Diagnostics, Winnersh, U.K.) instead of primary antibody.
Assessment of staining
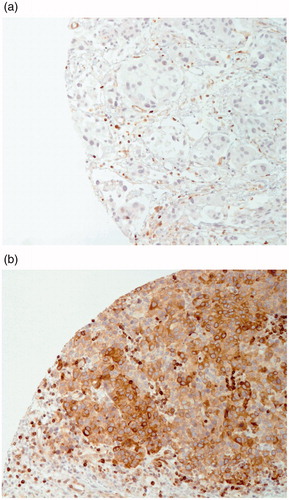
Staining quality and score criteria were determined by a board-certified pathologist (M.A.) with experience of Bcl-2 staining in routine daily practice. Two independent observers assessed all samples, and discordant data were evaluated by the pathologist, who made the final decision. For each patient, two different Bcl-2 stained T.M.A.s were evaluated for assessment of the cytoplasmic staining extension in terms of percentage area of positive staining. Staining intensity was not considered. Samples with a staining area of 10% or more were considered Bcl-2 positive, as previously described [Citation16,Citation19] ().
Statistical analyses
O.S. and disease-specific survival (D.S.S.) curves were constructed according to the Kaplan-Meier method, and differences between groups were tested using the log rank test. Univariate and multivariate Cox proportional hazards regression analyses were performed adjusting for patient inclusion in one of the two trials, treatment allocation to cystectomy with or without neoadjuvant chemotherapy, gender, age, T-category, and marker status. The interaction between neoadjuvant treatment and marker status was also tested for significance in Cox analyses. Chi-squared tests and unpaired t-tests were used to compare categorical and quantitative variables. Variables in the statistical analyses were defined as follows. Clinical staging was determined by T.U.R. and imaging studies, and pathological staging was determined by cystectomy pathology. Downstaging was defined as downstaging from clinical T2 or higher to pathological Ta, Tis, or T0 stages. Age was assessed as a continuous variable. Because there were some differences in the interventions between the two trials, trial group was also assessed as a categorical variable. For assessment of neoadjuvant chemotherapy response, both downstaging and survival ratios were calculated, but survival was used as response criteria. Statistical analyses were performed using version 20 of the I.B.M. S.P.S.S. software package.
Results
I.H.C. analysis was performed on tissue samples from 247 patients, 11 of whom were excluded after the staining procedure because of the low number of tumor cells on the T.M.A. sample. The clinicopathological properties of the remaining 236 patients are shown in .
Table 1. Clinicopathological characteristics of all 236 eligible patients (no statistical differences were detected between neoadjuvant and cystectomy groups).
Of the eligible tumor samples, 38% (n = 90) were assessed as Bcl-2 positive and 62% (n = 146) as Bcl-2 negative. The predictive value of Bcl-2 status was assessed by comparing patients treated with cystectomy combined with neoadjuvant chemotherapy (chemotherapy group) vs those treated only with cystectomy (no-chemotherapy group) both for Bcl-2 negative and positive patients. Among the Bcl-2 negative patients, the chemotherapy group had statistically better 5-year O.S. compared to the no-chemotherapy group (mean O.S.=43 months vs 33 months, respectively, p = 0.009), while no statistically significant difference was observed for the Bcl-2 positive patients (mean O.S. = 47 months vs 42 months, respectively, p = 0.4) ().
Figure 2. Kaplan-Meier overall survival curves for patients with or without neoadjuvant chemotherapy and different Bcl-2 status.
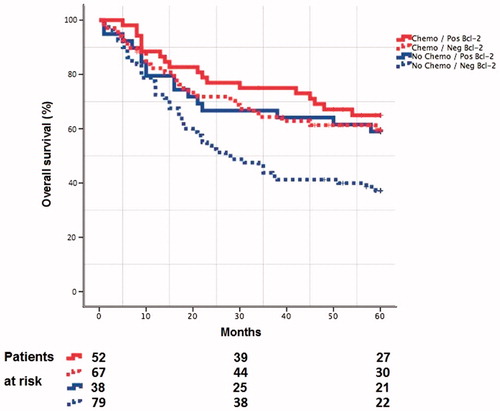
In addition, the interaction between Bcl-2 status and neoadjuvant treatment was not significant (p = 0.38).
Similar results were also obtained for 5-year D.S.S. for all of the above calculations (data not shown). Information for downstaging was available in 106 (89%) patients that received neoadjuvant cisplatin treatment. Downstaging was observed in 48% and 38% of Bcl-2 positive and negative patients, respectively (p = 0.3).
The prognostic value of Bcl-2 protein expression by tumor cells was evaluated in the no-chemotherapy group. Bcl-2 positive patients had significantly better 5-year O.S. compared to Bcl-2 negative patients (42 months vs 33 months, p = 0.04). This survival advantage was not independent when age, gender, trial, and clinical T-category variables were adjusted (H.R. = 1.69, 95% CI = 0.95–3.00, p = 0.07). When all the patients were analyzed in a multivariate Cox model including gender, age, trial, clinical T-category, and Bcl-2 status, Bcl-2 was not a significant independent factor ().
Table 2. Multivariate Cox regression analyses of all 236 patients evaluating associated co-factors for survival.
Discussion
Bladder cancer, with its high incidence and mortality rate, is regarded as a serious health threat around the world. Although a large number of studies have attempted to discover biological markers for better patient management, none have entered the clinical routine. Proper selection of patients for neoadjuvant chemotherapy is one problem. Studies involving more sophisticated methods such as gene signature techniques [Citation20,Citation21] show promise, but they are expensive and have no widespread utility yet. In contrast, I.H.C. has been used around the world in routine practice for many years, resulting in increased experience with application and assessment of these techniques.
In this study, we evaluated the predictive value of Bcl-2 expression on tumor cells for response to neoadjuvant cisplatin treatment, using I.H.C. to identify Bcl-2 protein status on tumor tissue. Our hypothesis was that Bcl-2 negative patients would have an enhanced neoadjuvant chemotherapy response and improved survival following neoadjuvant chemotherapy. To our knowledge, this is the largest study in the literature evaluating this specific topic of bladder cancer. Furthermore, patients were randomized into treatment and control arms. Having a control arm with no neoadjuvant treatment gave us the chance to determine if enhanced survival was related to neoadjuvant cisplatin treatment, or if these patients would in fact have a better prognosis without neoadjuvant treatment. Our data did not demonstrate Bcl-2 as a predictor of neoadjuvant cisplatin response. Although Bcl-2 status seemed related to neoadjuvant treatment response in the Kaplan Meier analysis, the interaction between Bcl-2 status and treatment was not significant. According to Ballman [Citation22], the interaction between the marker status (Bcl-2 status) and the treatment must be significant to prove that the marker has an independent predictive value.
Positive Bcl-2 status was correlated with improved survival for the cystectomy-only group in univariate analyses, but this relation did not remain independent in the multivariate analyses.
In bladder cancer, positive expression of Bcl-2 protein has been correlated with poor neoadjuvant cisplatin treatment response [Citation16,Citation23,Citation24]. One of the earlier clinical studies evaluated the predictive value of Bcl-2 among 51 patients with M.I.B.C. in a randomized trial of radiotherapy with or without cisplatin, and reported similar findings to ours. Bcl-2 negative patients receiving neoadjuvant chemotherapy had longer survival rates than Bcl-2 positive patients receiving the same treatment (median survival of 72 months vs 17 months) [Citation23]. The conclusion was that Bcl-2 status could identify patients who may benefit from neoadjuvant chemotherapy. Unfortunately, there was no information about the interaction of marker status and neoadjuvant treatment.
More recently, Kiss et al. [Citation16] studied the possible predictive effect of Bcl-2 and concluded that over-expression is related to poor chemotherapy response in bladder cancer and might help to select likely non-responders. Patients negative for Bcl-2 had a tendency towards a better response to neoadjuvant chemotherapy, but this did not reach statistical significance. In univariate and multivariate analyses, Bcl-2 had no significant prognostic information. The major limitation of that study was the low patient numbers for evaluation of the predictive value of Bcl-2.
Another study evaluated the prediction of neoadjuvant M.-V.A.C. (methotrexate, vinblastine, doxorubicin and cisplatin) response followed by definitive treatment for muscle-invasive and node-negative urothelial cancer in a retrospectively collected dataset of 59 patients [Citation24]. Univariate analyses showed no significant predictive value of Bcl-2 immunoreactivity. However, a decreased 5-year survival was reported when all three markers in their panel (mdm-2, p53, Bcl-2) had been over-expressed compared to normal expression (25% vs 54%). No association between chemotherapy response and marker status was found. The study was limited by the absence of a control group receiving no neoadjuvant chemotherapy.
Studies evaluating the prognostic value of Bcl-2 have reported conflicting results [Citation25]. Although one study found that a high level of Bcl-2 protein was associated with a poorer response to different treatment modalities in different cancer types [Citation26], the opposing view was also supported by studies correlating Bcl-2 positivity with improved prognosis [Citation12,Citation14].
Bcl-2 positivity was correlated with decreased D.S.S. (H.R. = 1.71, p = 0.013) for M.I.B.C. patients undergoing cystectomy [Citation27]. Conversely, positive expression of Bcl-2 has also been correlated with a favorable prognosis (H.R. = 0.179, p = 0.0474) in stage T2–4 invasive urothelial bladder cancer [Citation28]. Other studies failed to show any significant prognostic value of this protein [Citation16,Citation19,Citation23].
The major limitation of our study stems from methodological limitations attributed to I.H.C., such as subjective scoring and reliance on antibody quality [Citation29]. Another limitation is related to the patient cohorts. Preoperative radiotherapy was given to all patients in the first Nordic trial, but was not used in the second trial. To examine the impact of these limitations, we adjusted trial factors in multivariate analyses, and no significant effect was noted. In addition, the chemotherapy regimens used in these trials not only differed between the trials, but also are not in general use today. From these two trials we could only use Swedish patient tissue, but, since the trials were stratified by country, the risk of confounding was minimal. Lymph node status in the cystectomy specimen, which is considered one of the most important prognostic variables in M.I.B.C. today, was also not available in our material. Finally, it is important to mention that we evaluated only one protein in the Bcl-2 family. Investigation of different proteins from this family could be crucial.
Conclusions
According to the results of this study, Bcl-2 is neither an independent predictor of neoadjuvant chemotherapy response, nor a prognostic marker for M.I.B.C. patients.
| Abbreviations | ||
| M.I.B.C. | = | muscle-invasive urothelial bladder cancer |
| O.S. | = | overall survival |
| N.C.S. | = | Nordic collaborative study |
| T.U.R. | = | transurethral resection |
| F.F.P.E. | = | formalin-fixed paraffin-embedded |
| T.M.A.s | = | tissue microarrays |
| I.H.C. | = | immunohistochemistry |
| D.S.S. | = | disease-specific survival |
| M.-V.A.C. | = | methotrexate, vinblastine, doxorubicin and cisplatin |
Supplementary_Bcl-2_Figure_1.docx
Download MS Word (90.7 KB)Acknowledgments
We are grateful to pathologists Christer Busch (C.B.) and Maysaa Abdulsalam Abdulla (M.A.) for evaluating the tumor tissue and annotating the immunohistochemical staining, and to statistician Lisa Wernroth for skilful assistance with the statistical analysis. We are also grateful to the members of the Nordic Urothelial Cancer group for their contribution with follow-up and collection of cancer material.
Additional information
Funding
References
- Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol. 2006;24:296–304.
- Sherif A, Holmberg L, Rintala E, Nordic Urothelial Cancer Group, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45:297–303.
- Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. discussion 205-206.
- Witjes JA, Comperat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778–792.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866.
- McKnight JJ, Gray SB, O’Kane HF, et al. Apoptosis and chemotherapy for bladder cancer. J Urol. 2005;173:683–690.
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003;22:7265–7279.
- Niedner H, Christen R, Lin X, et al. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol. 2001;60:1153–1160.
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629.
- Farrow SN, Brown R. New members of the Bcl-2 family and their protein partners. Curr Opin Genet Dev. 1996;6:45–49.
- Kluck RM, Bossy-Wetzel E, Green DR, et al. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136.
- Zhao X-D, He Y-Y, Gao J, et al. High expression of Bcl-2 protein predicts favorable outcome in non-small cell lung cancer: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:8861–8869.
- Sekine I, Shimizu C, Nishio K, et al. A literature review of molecular markers predictive of clinical response to cytotoxic chemotherapy in patients with breast cancer. Int J Clin Oncol. 2009;14:112–119.
- Bouchalova K, Kharaishvili G, Bouchal J, et al. Triple negative breast cancer - BCL2 in prognosis and prediction. Review. Curr Drug Targets. 2014;15:1166–1175.
- Hemdan T, Malmstrom PU, Jahnson S, et al. Emmprin expression predicts response and survival following cisplatin containing chemotherapy for bladder cancer: a validation study. J Urol. 2015;194:1575–1581.
- Kiss B, Skuginna V, Fleischmann A, et al. Bcl-2 predicts response to neoadjuvant chemotherapy and is overexpressed in lymph node metastases of urothelial cancer of the bladder. Urol Oncol 2015;33:166.e161–168.
- Segersten MU, Edlund EK, Micke P, et al. A novel strategy based on histological protein profiling in-silico for identifying potential biomarkers in urinary bladder cancer. BJU Int. 2009;104:1780–1785.
- Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
- Rodel C, Grabenbauer GG, Rodel F, et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: possible predictors for response to radiochemotherapy and successful bladder preservation. Int J Radiat Oncol Biol Phys. 2000;46:1213–1221.
- Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol. 2015;68:959–967.
- Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. 2016;69:384–388.
- Ballman KV. Biomarker: predictive or prognostic? J Clin Oncol. 2015;33:3968–3971.
- Cooke PW, James ND, Ganesan R, et al. Bcl-2 expression identifies patients with advanced bladder cancer treated by radiotherapy who benefit from neoadjuvant chemotherapy. BJU Int. 2000;85:829–835.
- Maluf FC, Cordon-Cardo C, Verbel DA, et al. Assessing interactions between mdm-2, p53, and bcl-2 as prognostic variables in muscle-invasive bladder cancer treated with neo-adjuvant chemotherapy followed by locoregional surgical treatment. Ann Oncol. 2006;17:1677–1686.
- Gioacchini FM, Alicandri-Ciufelli M, Rubini C, et al. Prognostic value of Bcl-2 expression in squamous cell carcinoma of the larynx: a systematic review. Int J Biol Markers. 2015;30:e155–e160.
- Stein JP, Grossfeld GD, Ginsberg DA, et al. Prognostic markers in bladder cancer: a contemporary review of the literature. J Urol. 1998;160:645–659.
- Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8:128–136.
- Uchida T, Minei S, Gao JP, et al. Clinical significance of p53, MDM2 and bcl-2 expression in transitional cell carcinoma of the bladder. Oncol Rep. 2002;9:253–259.
- Anagnostou VK, Welsh AW, Giltnane JM, et al. Analytic variability in immunohistochemistry biomarker studies. Cancer Epidemiol Biomarkers Prev. 2010;19:982–991.