Abstract
Background: Transurethral resection of the prostate (TURP) is ‘Gold Standard’ treatment for moderate-to-severe lower urinary tract symptoms (LUTS) due to benign prostate hyperplasia (BPH) with bladder-outlet obstruction (BOO). However, TURP is associated with a risk of complications, so minimally invasive methods have been developed. Prostate artery embolization (PAE) is a new minimally invasive procedure. This study reports the outcomes of PAE when introduced in a ‘real life’ clinical setting in a Swedish County hospital.
Methods: A prospective, single-center, single-arm study in a consecutive vascular-anatomy ‘all comers’ population, eligible for TURP or adenomaenukleation, but unsuitable for this, treated with PAE from January 2015 to June 2018. Defined improvement of IPSS/QoL scores, or freedom from urinary catheter if previous urinary catheter-dependent, or clean intermittent catheterization (CIC) were considered as clinically successful treatments. PAE was performed until arterial stasis using the Perfected technique. Most patients were treated during a day-care procedure.
Results: Of 37 treated men, bilateral PAEs were achieved in 32 patients, unilateral PAEs in four patients, and bilateral failure in one patient due to difficult vascular anatomy. Clinically successful treatment was achieved in 84%, without serious adverse events.
Conclusions: PAE was introduced in Sweden, showing PAE as a novel and good minimally invasive alternative in treatment of symptomatic BPH, possible to perform as a day-care procedure.
Introduction
Lower urinary tract symptoms (LUTS) due to benign prostate hyperplasia (BPH) with bladder-outlet obstruction (BOO) are very common with increasing age [Citation1,Citation2]. About 30% of all men aged 50–80 years are affected [Citation1,Citation2]. Many patients are treated medically, but 30% need surgery because of suboptimal or side-effects of the given medication.
The most accepted ‘Gold Standard’ treatment for moderate-to-severe LUTS, not responding to pharmacological treatment, is transurethral resection of the prostate (TURP) or open or laparoscopic adenoma enucleation for prostates over 80–90 cc [Citation3,Citation4].
TURP, however, is associated with a cumulate risk of complications of 11%. The most common complications are post-operative bladder dysfunction (5%), risk for reoperation (5%), bleeding (3%) correlating with the amount of resected prostate tissue, urinary infections (4%) and the risk of erectile dysfunction (6, 5%) [Citation1,Citation5,Citation6]. Open surgery with adenoma enucleation is associated with an even higher risk of complications and prolonged hospitalization.
Many minimal invasive methods for treatment of symptomatic BPH have been developed, such as transurethral microwave treatment (TUMT), Aqua ablation (Rezūm), Uro-lift, Holmium, and Green lasers. These minimally invasive methods have different limitations; e.g. impaired results in cases with large prostate size or presence of a lobus tertius. In addition, they are invasive to the urinary system and often imply investments in costly equipment [Citation7]. Some of these methods also lack long-term results.
Prostate artery embolization (PAE) is a new minimally invasive procedure [Citation8], non-invasive to the urinary system and possible to perform as day-care treatment.
The sections for Urology and Interventional Radiology in Helsingborg, Sweden, have long experience of multidisciplinary co-operation. We introduced PAE as the first Swedish center in the armamentarium of treatments of patients with LUTS caused by BPH and report the first Swedish outcomes of PAE when introduced as vascular-anatomy ‘all comers’ treatment in a ‘real life’ clinical setting in a Swedish County hospital.
Materials and methods
PAE technique
The PAE treatments were performed without pre-operative CT or MR angiography.
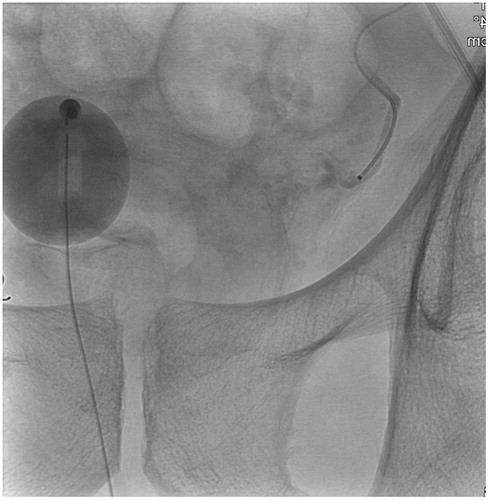
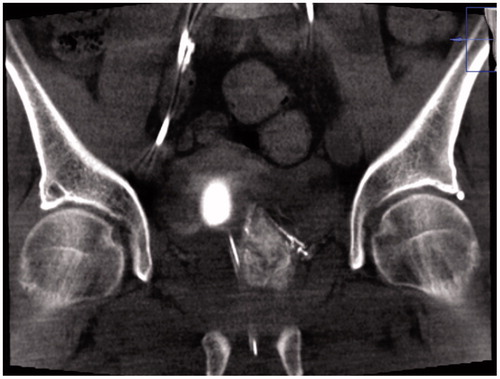
A Foley balloon placed in the bladder was filled with 10 ml iodinated contrast medium (Visipaque 140 mg I/ml mixed with 50% saline solution) in order to visualize the level of the arteries to the prostate gland. In an interventional radiology angio suite (FD20 digital subtraction angiography unit; Philips, Best, The Netherlands) in local anesthesia, through right femoral approach, selective DSA with non-ionic contrast material (Visipaque 140 mg I/ml; GE healthcare, Chicago, Illinois, USA) of both iliac arteries, followed by catheterization with 4 F glide cobra catheter (Terumo, Tokyo, Japan) and catheterization of both inferior vesical arteries with micro catheters (Progreat 2.0, Terumo, Tokyo, Japan) (). Selective angiogram with 3–5 ml contrast medium to visualize the blood supply to the prostate and Cone-Beam CT (CBCT) to ensure proper placement of the micro catheter tip in the prostate artery () was performed.
Figure 1. Angiography of left inferior vesical artery, in Prostate Artery Embolization (PAE) treatment.
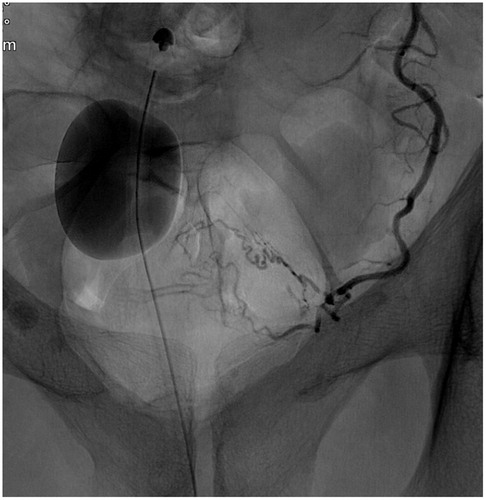
Figure 2. Cone-beam CT (CBCT) in coronal projection, showing contrast filling of prostate parenchyma and, thereby, correct catheter position prior to injection of particles in Prostate Artery Embolization (PAE) treatment.
One vial of Embosphere 300–500 (Merit Medical, Salt Lake City, UT) was diluted with 15 ml contrast medium (Visipaque 270 mg I/ml). The mixture was injected slowly during fluoroscopy until arterial stasis, using the Perfected technique (), with the microcatheter initially in a proximal position of the inferior vesical artery, then with the microcatheter advanced to a more distal position closer to the prostate gland, aiming for necrosis and volume reduction of the prostate gland [Citation9]. The patients were discharged from the day-care unit after 4 hours observation (range 3–6 hours) which ensured hemostasis of the closure device in the common femoral artery and adequate function after urinary catheter withdrawal (chronic catheter users had the catheter removed after 1 week).
Outcome measure
In the absence of a clear international definition of successful BPH treatment, we use a composite variable for clinically successful treatment [Citation10]; reduction of IPSS score of at least 25% (and QoL ≤ 3), or improvement of QoL of 3 points, or freedom from urinary catheter in patient with previous chronic use or clean intermittent catheterization (CIC), and urinary flow > 10 ml/s.
Population
This prospective, single-center, single-arm study was conducted in all men consecutively treated with PAE from January 2015 to June 2018. Patients had large prostates (a majority > 90 cc measured by preoperative Trans Rectal Ultrasound (TRUS) and met the indications for operation with TURP or adenoma enucleation. All patients who were to be treated with PAE were clinically eligible for TURP/adenoma enucleation but, due to concurrent heart and/or pulmonary disease, they were considered unsuitable for conventional operation or other minimal-invasive treatment (many patients had a large prostate with a lobus tertius). A few patients were selected for PAE due to their own preference for the method. (e.g. not accepting blood-transfusion). Chronic renal failure was considered contraindication [Citation11]. All patients were investigated according to the Swedish National guidelines [Citation12] for diagnosis of prostate cancer and EUA-guidelines [Citation1] regarding LUTS, including benign prostatic obstruction (BPO). They were all examined with Prostate Specific Antigen (PSA), TRUS and prostate palpation. None of the PAE-treated patients was considered to be at risk of significant prostate cancer. No undecided case was treated by PAE.
Treatment indications, minor and major 30 days treatment complications were prospectively recorded and were, together with 3 and 12-month follow-up data, collected retrospectively from the clinical records. A complication was considered as minor (Clavien 1–2) if it did not influence the treatment result and did not prolong hospitalization. Examples of minor complications are hematoma in the groin, post-embolization syndrome (perineal burning sensation with/without gross hematuria) or uncomplicated urinary infection. A complication with a Clavien 3–5 grade was regarded as a major complication.
Ethics
The study was performed in accordance with the spirit of the Declaration of Helsinki and in agreement with the guidelines for conducting a clinical investigation as outlined in ISO 14-155. Informed consent was obtained from all patients. The study was approved by the Ethics Committee at Lund University (Ref. no. 2013/786).
Statistics
Student’s T-test was used to evaluate differences in continuous variables, and the Chi2 test was used to evaluate differences in nominal variables between groups. Descriptive data are presented with mean/median and range. Calculations were performed using SPSS18.0 (SPSS Inc., Chicago, IL). Results were considered statistically significant at p < 0.05.
Results
The median age was 73 (56–90) years. Before treatment, 14 (38%) patients were urinary catheter-dependent, and five (13%) performed CIC (). Median pre-operative prostate volume was 92 (40–300) cc, median pre-operative urinary flow was 7 (1–14) ml/s and median residual volume was 200 (12–2100) ml. In 17 patients without pre-operative urinary catheter, median IPSS was 21.2 (8–34) and QoL was 4.6 (3–6). Bilateral PAE was achieved in 32 patients (84%). In one patient (3%), the inferior vesical artery was impossible to intubate bilaterally, due to tortuous, calcified and narrow vessels and four patients (11%) received unilateral embolization for the same reason. All patients had a follow-up at 3 months and 16 patients had a follow-up at 12 months. The mean follow-up time in the cohort was 6.9 months. Of the 14 urinary catheter-dependent patients at baseline, only three patients were catheter users at follow-up. Of five patients with CIC at baseline only one patient used this treatment at follow-up (). Median urinary flow improved from 7 (1–14) to 13 (6–40) ml/s, and median residual urine improved from 200 (12–2100) to 100 (0–300) ml at follow-up. Significant median IPSS and QoL improvements were reported (). According to the composite outcome variable clinically successful treatment was achieved in 31 (84%) cases. The improvement was stable in all patients but one, who experienced LUTS recurrence after 8 months. Five patients did not improve (), and one patient declined follow-up. No major PAE treatment-related complication was noted, but two (5%) minor complications occurred (). No patients were treated by alpha-blocker or 5α-reductase inhibitors after the PAE.
Table 1. Baseline data in patients (n = 37) treated with prostate artery embolization (PAE). Mean (SD) or N (%).
Table 2. Patients (n = 37) urinary catheter characteristics before and after treatment with prostate artery embolization (PAE).
Table 3. Changes in IPSS and QoL in patients without urinary catheter at baseline (n = 17) treated with prostate artery embolization (PAE).
Table 4. Characteristic in patients (n = 5) with clinical failure after treatment with prostate artery embolization (PAE).
Table 5. Complications in patients (n = 37) treated with prostate artery embolization (PAE).
Discussion
We report good results when introducing PAE in the clinical setting, with interventional radiology and urology in close co-operation, in a Swedish county hospital. Clinically successful treatment in 84% without serious adverse events in this study is comparable with results reported in the literature [Citation13–15], despite a selection-bias in our study with clinically unfavorable and anatomically difficult cases.
Parallel to this study, a number of studies have been performed throughout the world. The results have been equal, with successful treatment in 85% [Citation16,Citation17]. A prospective randomized study of PAE vs TURP has been performed in Great Britain [Citation17], its results have led to the National Health Service recommending PAE treatment since spring 2018.
The use of CBCT as an adjuvant technique provides useful information and helps treatment planning during PAE, to avoid non-target embolization [Citation18], which may have played an important role in the fact that no major complications occurred in this study. The minor complications, one PAE-syndrome and one diarrhea, are similar to those reported in other studies [Citation13–16]. PAE-syndrome is a burning sensation in the perineum, sometimes accompanied by discomfort or slight fever for a couple of days, affecting ∼ 9% of patients [Citation13–16], and can be effectively alleviated with paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs). Urinary infection may occur after PAE, as an indwelling catheter is used during the procedure. This was, however, not seen in this study.
Bilateral PAEs are possible in most cases, but technical failures are reported in large materials; bilateral failure in 2% and unilateral failure in 7.5% [Citation16]. We report four cases with unilateral PAE treatment. It is interesting to note that this does not necessarily lead to clinical failure. Clinically successful treatment in three of these patients is in line with other studies that do not report any statistically significant difference between uni and bilateral PAE treatment [Citation19].
PAE is not an immediate ablative technique. PAE destroys the prostate vasculature and more than a month is needed for the prostate to complete its complicated histopathological changes. An early atrophy of prostate tissue due to ischemia is achieved, later accompanied by a prostatic atrophy due to q decrease of intraprostatic conversion of the testerone into dehydrotestosterone due to PAE-induced atrophy of 5-alpha reductase expression cells [Citation20]. This is why we choose to delay the removal of the urinary catheter in the more severe cases, i.e. the catheter users. This may also be the reason why PAE does not have the early, often occurring distressing symptoms (urinary retention and pain) due to prostatic inflammation with swelling, lasting for some month, after other more immediate ablative methods like TUMT.
A clinical success rate of 84% is consistent with other reports. In examining the clinical failures in this study, we find the following; one patient with bilateral failed PAE apparently received no treatment. One patient with unilateral treatment had a small prostate (50 cc). Three patients with technically successful PAE treatments did not improve clinically; one patient with a 190 cc prostate shrinking to 65 cc still had to continue with CIC. The clinical outcome after PAE might be better in patients with larger prostate glands than in patients with medium-sized ones [Citation21], and two patients with technically successful PAE treatments in this study did not improve clinically, without any other obvious reason other than small prostate volumes (40–50 cc).
A limitation in this study is the rather short study time that can give rise to questions about the treatment’s durability. It is, however, interesting to note that most failures after PAE seem to appear early after treatment, and the durability of PAE treatment has been demonstrated in a large study showing median (3–5 years) and long-term (> 5 years) results of 81.9% and 76.3% successful treatment, respectively [Citation16].
This study reports all consecutive PAE cases during the study period, which can be considered as both strengths and limitations. Many reports on PAE are performed as trials with narrow inclusion and exclusion criteria, excluding cases with e.g. advanced tortuosity or arteriosclerosis in pelvic vessel found on pre-operative CT or MR angiography. This study shows the results when introducing PAE into clinical practice without pre-operative imaging as a vascular-anatomy ‘all comers’ trial, making the results reproducible into ‘real life’ clinical settings.
The possibility of treating BPH patients in day-care and discharging them from the hospital on the same day is another great advantage in relation to the rather constraining situation regarding hospital beds in the Swedish healthcare system.
We have noted a great interest from other Swedish hospitals to introduce the method and are involved in the training in some of these. It will be interesting to follow the development of PAE in Sweden.
Conclusion
Results when introducing PAE in Sweden were appealing, showing PAE as a good minimally invasive alternative in treatment of symptomatic BPH. It is possible to perform as a day-care procedure.
Acknowledgments
This study has been possible through the generous support from the Stig and Ragna Gorthon’s foundation for medical research.
Disclosure statement
M. Bläckberg has nothing to disclose. H. Lindgren has received compensation according to a proctoring and training agreement with Ev3 Nordic AB, William Cook Europe ApS, and Merit Medical Systems AB. These companies had no involvement in any part of the study. The study was conducted without sponsoring from any medical device company.
References
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–1109.
- Logie J, Clifford GM, Farmer RD. Incidence, prevalence and management of lower urinary tract symptoms in men in the UK. BJU Int. 2005;95:557–562.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803.
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU Guidelines on the Management of non-neurogenic male lower urinary tract symptoms (LUTS), including benign prostatic obstruction (BPO). Eur Urol. 2015;67(6):1099–1109
- Reich O, Gratzke C, Bachmann A, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol. 2008;180:246–249.
- El-Assmy A, ElShal AM, Mekkawy R, et al. Erectile and ejaculatory functions changes following bipolar versus monopolar transurethral resection of the prostate: a prospective randomized study. Int Urol Nephrol. 2018;50:1569–1576.
- Sievert KD, Kunit T. Emerging techniques in 'truly' minimal-invasive treatment options of benign prostatic obstruction. Curr Opin Urol. 2017;27:287–292.
- Wang XY, Zong HT, Zhang Y. Efficacy and safety of prostate artery embolization on lower urinary tract symptoms related to benign prostatic hyperplasia: a systematic review and meta-analysis. CIA. 2016;11:1609–1622.
- Carnevale FC, Moreira AM, Antunes AA. The "PErFecTED technique": proximal embolization first, then embolize distal for benign prostatic hyperplasia. Cardiovasc Intervent Radiol. 2014;37:1602–1605.
- Pereira J, Bilhim T, Duarte M, et al. Patient selection and counseling before prostatic arterial embolization. Tech Vasc Interv Radiol. 2012;15:270–275.
- Antunes AA, Carnevale FC, da Motta Leal Filho JM, et al. Clinical, laboratorial, and urodynamic findings of prostatic artery embolization for the treatment of urinary retention related to benign prostatic hyperplasia. A prospective single-center pilot study. Cardiovasc Intervent Radiol. 2013;36:978–986.
- Confederation of Regional Cancer Centres in Sweden/The Swedish national clinical prostate cancer care guidelines. https://www.cancercentrum.se/samverkan/cancerdiagnoser/prostata/vardprogram/gallande-vardprogram-prostatacancer/
- Rampoldi A, Barbosa F, Secco S, et al. Prostatic artery embolization as an alternative to indwelling bladder catheterization to manage benign prostatic hyperplasia in poor surgical candidates. Cardiovasc Intervent Radiol. 2017;40:530–536.
- Gao YA, Huang Y, Zhang R, et al. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate–a prospective, randomized, and controlled clinical trial. Radiology. 2014;270:920–928.
- Uflacker A, Haskal ZJ, Bilhim T, et al. Meta-analysis of prostatic artery embolization for benign prostatic hyperplasia. J Vasc Interv Radiol. 2016;27:1686–1697.
- Bhatia S, Sinha VK, Harward S, et al. Prostate artery embolization in patients with prostate volumes of 80 mL or more: a single-institution retrospective experience of 93 patients. J Vasc Interv Radiol. 2018;29:1392–1398.
- Ray AF, Powell J, Speakman MJ, et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int. 2018;122:270–282.
- Wang MQ, Duan F, Yuan K, et al. Benign prostatic hyperplasia: cone-beam CT in conjunction with DSA for identifying prostatic arterial anatomy. Radiology. 2017;282:271–280.
- Sun F, Crisóstomo V, Báez-Díaz C, et al. Prostatic Artery Embolization (PAE) for Symptomatic Benign Prostatic Hyperplasia (BPH): part 2, insights into the technical rationale. Cardiovasc Intervent Radiol. 2016;39:161–169.
- Sun F, Sánchez FM, Crisóstomo V, et al. Transarterial prostatic embolization: initial experience in a canine model. AJR Am J Roentgenol. 2011;197:495–501.
- Wang M, Guo L, Duan F, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms caused by benign prostatic hyperplasia: a comparative study of medium- and large-volume prostates. BJU Int. 2016;117:155–164.