Abstract
Objective: TRUS-guided prostatic biopsies are the mainstay procedure to diagnose prostatic cancer. The aim was to investigate how accurate and reliable these biopsies are by comparing them with the final pathology results after prostatectomy.
Materials and methods: One hundred consecutive patients diagnosed with localized prostatic cancer using this technique and who subsequently underwent a radical prostatectomy in Västerbotten County were included in this study. From the pathological-anatomical diagnosis (PAD) of core needle biopsies, data was extracted on the location of the tumour within the prostate, the tumour volume and the Gleason score, and compared with the characteristics of the prostatectomy specimen. The frequency and type of deviation between the pre-operative and post-operative examinations was recorded.
Results: In 95% of the cases there was a poor correlation between the pre-operative and post-operative pathological reports. In the final report, 48% had a higher Gleason score and 88% had deviations in localization when compared with the information from the biopsies. If known prior to surgery, a total of 104 of these deviations might have had a significant impact on the surgical strategy.
Conclusions: The pre-operative biopsies in this setting rarely match the final prostate PAD results (5%). The most common deviations were in localization and in Gleason score, where the majority consisted of a higher Gleason score and/or tumour presence in a previously unknown location. This information, if known prior to surgery, might have altered the treatment strategy and ultimately the outcome of the treatment.
Introduction
In Sweden and many other countries, transrectal ultrasound (TRUS) guided prostatic biopsies are taken at 10–12 standardized locations when there is suspicion of prostate cancer [Citation1]. The amount of tissue obtained from the biopsies is only a small part of the prostate and can, therefore, miss potential crucial pathological information. These biopsies play a significant role in determining the tumour characteristics, treatment choices and prognosis [Citation1]. For example; whether to preserve the neurovascular bundle or do a more extensive radical surgery, sometimes with lymph node staging. In a clinical setting, there appears to be a significant discrepancy between the pre-operative biopsies and the final post-operative microscopic report of the removed specimen [Citation2–5].
Several studies have raised concerns of the TRUS systematic 12-core biopsy (SYS) technique. Serefoglu et al. [Citation3] found that the detection rate was low. Tei et al. [Citation4] concluded that approximately half the significant cancers were not adequately detected. Another study demonstrated that the accuracy of biopsies was decreased in certain locations of the prostate, and concluded that more biopsies should be taken in those areas [Citation5].
Chambó et al. [Citation6] concluded that there is no significant difference between the 12-core biopsies when compared to 10-core and 16-core biopsy protocols, although Miyoshi et al. [Citation7] found that, overall, the 16-core biopsy group had a slightly higher detection rate.
Furthermore, magnetic resonance imaging targeted (MRT-targeted biopsies) vs SYS have been compared. MRT-targeted biopsy, when added to SYS, was associated with a higher detection rate of clinically significant cancer in biopsy-naive patients when compared to SYS alone [Citation8]. Siddiqui et al. [Citation9] found that MRT/Ultrasound-fusion-guided biopsy upgrades and detects prostate cancer of higher Gleason grade in 32% of patients when compared to 12-core biopsy alone. MRT-targeted biopsy reduces the detection of clinically insignificant disease, while increasing the efficiency of detection of high-risk prostate cancer [Citation10].
Purpose
The purpose of this study was to examine and compare the results of pre-operative biopsies of the prostate with the final pathology results after prostatectomy in Västerbotten County. In addition to compare our results with results reported in other studies. Finally, to evaluate, based on the results, if a change of current routines should be considered.
Materials and methods
A retrospective review of CNB and prostatectomy PAD in 100 consecutive patients (mean age = 67, range = 45–78) who had been diagnosed with prostatic cancer (C61.9), who subsequently underwent a radical prostatectomy (KEC01) at Surgery Centrum in Västerbotten County between 1 January 2016 and 31 December 2017, was conducted for this study. This cohort consisted of patients resident in Västerbotten County, diagnosed and treated at Norrlands University Hospital. Two patients were excluded from the study due to inconclusive final PAD reports.
The material was obtained from medical records, including laboratory and pathological reports. The data collected included information on PSA, digital rectal examination (DRE), transrectal ultrasound findings (TRUS), tumour volume, tumour location and Gleason score from both the CNB and the final PAD report.
In this study a deviation is defined as a discrepancy between biopsy and post-operative PAD results in localization and/or Gleason grade. For example; if the biopsies of the apex in the left prostate lobe did not contain any tumour, but PAD results revealed there was tumour tissue present, those biopsies will be regarded as deviations. The localizations were defined as the left and right lobe and the apex, mid-prostate and base on each side. Furthermore, a discrepancy in the assessed Gleason grade following biopsies compared with PAD results was counted as a deviation if it was higher or lower. The total deviations in each patient was recorded as well as the type of deviations (localization and Gleason grade).
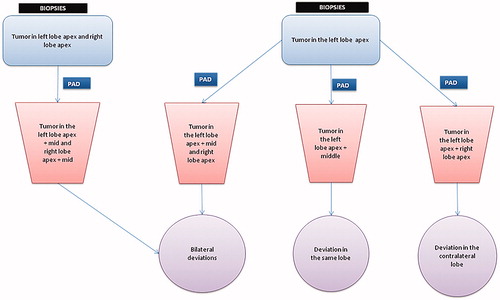
Deviations in localization of tumour were defined as follows; if biopsies showed a tumour in the left apex, but the PAD results also found tumour tissue in the mid or base in the same lobe, it was recorded as a deviation in the same lobe. If the biopsies showed a tumour exclusively in the left lobe, but the PAD results also found a tumour in the right lobe, it was defined as a deviation in the contra-lateral lobe. Bilateral deviations mean there are discrepancies in both lobes when comparing biopsies with PAD results, e.g. biopsies found tumors in the left lobe apex and right lobe apex, but PAD results additionally found tumour tissue in the mid-prostate in both lobes. If the biopsies found tumour tissue in only one part of one lobe, and PAD results show the tumour in an additional part of the same lobe as well as in the opposite lobe, it would count as bilateral deviations ().
The different types of deviations were divided into groups with the aim to stratify their relevance by comparing the proportion of deviations in localization of the prostate between biopsies and PAD results, and also the discrepancy in Gleason score.
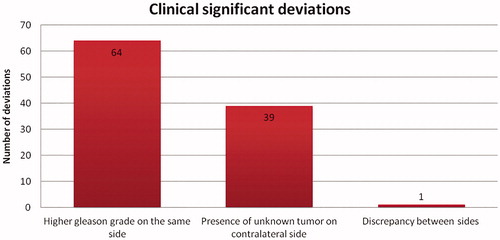
Clinically significant deviations were defined as deviations that might lead to a different treatment strategy if known prior to surgery. If there was a higher Gleason grade in the same lobe after the PAD results, if there was an unknown tumour present in the contralateral lobe, or if there was a discrepancy between sides such that a tumour exclusive to the right lobe was in fact exclusive to the left lobe, these were considered to be clinically significant deviations.
Ethics
This is a retrospective quality assurance review, that does not contain any identifying information and was conducted on data gathered from already diagnosed and managed patients whom will not be affected by the study. Furthermore, as all patients are anonymized and this study does not require informed consent, a formal ethical approval is not mandatory and, thus, was not applied for. The study follows the principles of the Declaration of Helsinki.
Results
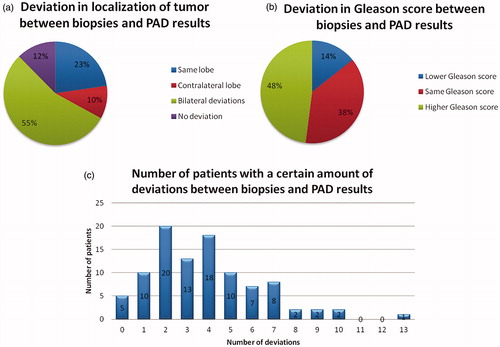
The data revealed that 95% of the patients had deviations between biopsies and PAD results after prostatectomy ().
Time between biopsies and prostatectomy
The mean time between biopsies and prostatectomy was 175 days (42–628) and the median time was 149 days.
Clinical assessment compared with PAD results
Comparing the clinical assessment (DRE and TRUS) with PAD results of the prostate shows a high discrepancy (). Ninety-five patients were assessed as a T0/T1/T2 graded tumour by clinical examination, but only 37 patients had a T0/T1/T2 graded tumour when examining the prostate after prostatectomy. The remaining 61 patients had a pT3 graded tumour.
Table 1. Characteristics and frequency of deviations on the post-operative PAD report that might have changed the pre-operative assessment of surgical strategy if known prior to surgery.
Out of the group of patients assessed with a T0/T1c tumour (n = 58) by clinical examination, 57% had a pT3 tumour after prostatectomy. When analysing the data of patients assessed to have a T2 tumour (n = 38), 66% had in fact a pT3 tumour. In total only in three cases the patient was assessed to have a T3 tumour pre-operatively but on the final PAD report after surgery 61 patients had a pT3 tumour.
The total amount of deviations among patients and clinically significant deviations
The number of deviations for each patient varied from none to 13. In 14% (n = 14) there was a downgrade in Gleason score. The most frequent clinically significant deviation, 36% (n = 64), was a higher Gleason grade on the same side followed by the presence of an unknown tumour on the contralateral side ( and ).
Discussion
The poor correlation between biopsies and PAD results after prostatectomy in our clinical setting is a matter for concern, particularly as a significant number of these deviations might have had a substantial clinical impact. If known prior to surgery, treatment strategies may have been different, leading to ultimately a better survival prognosis for these patients. Similar concerning results have been reported in other studies. A study analysing the diagnostic performance of 12-core biopsy in detecting significant prostate cancer concluded that approximately half of the significant cancers were not accurately detected [Citation4]. Sinnott et al. [Citation11] reported a discrepancy in prostate cancer localization between biopsy and prostatectomy specimens in patients with unilateral positive biopsy, it was concluded that 12-core biopsy is inadequate to identify candidates for organ-sparing therapy. Most men in this study had bilateral cancer at prostatectomy and tumours missed by biopsy were clinically significant in 40% of patients. Our study presents similar results, with 40% (39/98) of cases having the presence of unknown tumour on the contralateral side.
Regarding upgrade in Gleason score, Suer et al. [Citation12] found that an upgrade in Gleason score between biopsy and radical prostatectomy specimen occurred in 29% of cases compared to our results of 48% (47/98). Furthermore, another retrospective study found that overall 35% of the patients had an upgraded Gleason score after radical prostatectomy [Citation13].
The clinical assessment of the prostate seems to be of little help in determining the grade of the tumour, considering the high frequency of T3 graded tumours in patients clinically assessed at a lower grade. The results reveal that there is only a small difference between clinically assessed T0/T1c tumours and T2 tumours that are actually a pT3 graded tumour. A review that compared the digital rectal examination with the radical prostatectomy specimen presented a 36% incidence of extracapsular tumour extension and 31% of positive surgical margins, on the side assessed as clinically benign [Citation14].
What are the reasons behind the seemingly flawed method of TRUS guided biopsies of the prostate?
The reasons for these deviations are likely multifactorial. One reason could be poor technique in performing the biopsies based on inadequate training in or lack of understanding of the procedure.
Furthermore, the prostate biopsies cover only a small amount of tissue of the whole prostate. Therefore, it is easy to miss tumours. The prostate volume appears to play a part in the detection of prostatic cancer and correct Gleason score. Prostatic cancers detected in men with prostatic enlargement requiring multiple biopsies are more likely to be low-grade, low-volume tumours at final pathology, than those in men without prostate enlargement [Citation15]. Cancers in patients with smaller prostate volumes were at increased risk for Gleason score upgrade after radical prostatectomy [Citation13].
The written PAD report can sometimes be hard to interpret, and that can affect the results not only of this study. However, given the large number of deviations found in our study, this is not likely to have an impact on our result.
The time between biopsies and prostatectomy in this study is substantial, but is unlikely to have any significant impact on results, as in general prostate cancer has a slow progression over time.
From the findings of this and other studies, we conclude that TRUS guided biopsies and clinical examination (DRE) will not give us the robust data we need to make a correct clinical assessment in order to devise an appropriate treatment strategy, as these data are misleading in almost 50% of cases.
Magnetic resonance imaging of the prostate might be a method that can enhance diagnostics, particularly if this is done in connection with the biopsies. It may be able to detect more potentially serious cancers, detect less low risk cancers and reduce unnecessary biopsies [Citation8–10,Citation16,Citation17].
Based on the results presented it could be argued that a different approach to secure optimal treatment results would be to do more extensive surgery even for tumours classified as low grade on the CNB, minimize nerve sparing surgery, thereby not jeopardizing oncological results. With the current information it might be important to highlight to patients the risks of not conducting extensive surgery, since the distribution and development of prostatic tumours tend to be hard to predict pre-operatively with current standardized biopsy techniques [Citation18,Citation19].
Conclusion
The pre-operative biopsies do not match the final prostate PAD in the majority of cases. A significant number of these deviations have clinical significance and if known prior to surgery might have altered the treatment strategy and the post-operative oncological outcome. Hence, pre-operative CNB must be evaluated with great caution when assessing the surgical treatment strategy. Better pre-operative tools to assess prostate cancer characteristics are warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- https://www.cancercentrum.se/samverkan/cancerdiagnoser/prostata/vardprogram/gallande-vardprogram-prostatacancer/.
- Areal Calama J. [Conventional transrectal ultrasound guided biopsy. Current role, indications, techniques and limitations]. Arch Esp Urol. 2015;68:282–295.
- Serefoglu EC, Altinova S, Ugras NS, et al. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2013;7:E293–E298.
- Tei H, Miyake H, Harada K, et al. Detection of significant prostate cancer according to anatomical areas of sampling cores obtained with transrectal systematic 12-core biopsy. Curr Urol. 2014;8:91–95.
- Iremashvili V, Pelaez L, Jorda M, et al. Prostate sampling by 12-core biopsy: comparison of the biopsy results with tumor location in prostatectomy specimens. Urology. 2012;79:37–42.
- Chambó RC, Tsuji FH, de Oliveira Lima F, et al. What is the ideal core number for ultrasound-guided prostate biopsy? Korean J Urol. 2014;55:725–731.
- Miyoshi Y, Furuya M, Teranishi J, et al. Comparison of 12- and 16-core prostate biopsy in japanese patients with serum prostate-specific antigen level of 4.0–20.0 ng/mL. Urol J. 2014;11:1609–1614.
- Bladou F, Fogaing C, Levental M, et al. Transrectal ultrasound-guided biopsy for prostate cancer detection: systematic and/or magnetic-resonance imaging-targeted. CUAJ. 2017;11:E330–E337.
- Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–719.
- Weiss B, Loeb S. MRI/Ultrasound fusion biopsy versus standard 12-core biopsy. Rev Urol. 2015;17:113–115.
- Sinnott M, Falzarano SM, Hernandez AV, et al. Discrepancy in prostate cancer localization between biopsy and prostatectomy specimens in patients with unilateral positive biopsy: implications for focal therapy. Prostate. 2012;72:1179–1186.
- Suer E, Gokce MI, Gulpinar O, et al. How significant is upgrade in Gleason score between prostate biopsy and radical prostatectomy pathology while discussing less invasive treatment options? Scand J Urol. 2014;48:177–182.
- Chung MS, Lee SH, Lee DH, et al. Is small prostate volume a predictor of Gleason score upgrading after radical prostatectomy? Yonsei Med J. 2013;54:902–906.
- Obek C, Louis P, Civantos F, et al. Comparison of digital rectal examination and biopsy results with the radical prostatectomy specimen. J Urol. 1999;161:494–498.
- Pietzak EJ, Resnick MJ, Mucksavage P, et al. Multiple repeat prostate biopsies and the detection of clinically insignificant cancer in men with large prostates. Urology. 2014;84:380–385.
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–450.
- Demirel HC, Davis JW. Multiparametric magnetic resonance imaging: overview of the technique, clinical applications in prostate biopsy and future directions. Turk J Urol. 2018;44:93–102.
- Keulers BJ, Scheltinga MR, Houterman S, et al. Surgeons underestimate their patients' desire for preoperative information. World J Surg. 2008;32:964–970.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008 ;358:1250–1261.