Abstract
Objective: To assess urinary tract function and complications in a regional prevalence group of patients with traumatic spinal cord injury (SCI), and to estimate risk factors for recurring complications.
Materials and methods: A total of 412 patients who attended a yearly check-up at the Spinalis SCI clinic were included. A regional follow-up programme for neurogenic bladder dysfunction was applied, including S-creatinine and S-cystatin-C, urine culture, residual urine, ultrasound of kidneys, urodynamic studies, and a questionnaire regarding complications during the preceding year. Descriptive statistics and logistic regression were used to estimate risk factors.
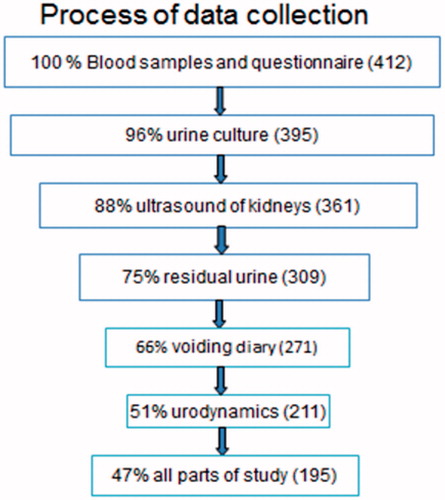
Results: All patients completed blood tests and the questionnaire. A urine culture was completed by 96%, ultrasound by 88%, residual urine by 75%, urodynamics by 51%, and all parts of the study by 47%. One quarter of patients displayed pathological findings regarding kidney function. Urodynamics verified neurogenic overactive bladder in a majority, and a high proportion with intravesical filling pressures above 40 cm H2O, a commonly used cut-off level for kidney safety. Subjectively, 47% of patients reported complications during the past year with urinary tract infection (UTI) as the most common one. Other complications were rare.
Conclusions: With the aid of a regular follow-up programme, SCI patients can achieve a relatively stable situation regarding urinary tract function. UTI is the most common complication. Indicators of renal complications are frequent but not clearly related to the number of UTIs, nor to intravesical filling pressures. Main risk factors for complications are cervical levels and more complete neurological lesions.
Introduction
This is the first article in a series describing the Stockholm Spinal Cord Uro Study, a comprehensive survey of urinary tract function in a regional prevalence population of patients with chronic traumatic spinal cord injury (SCI).
Historically, deteriorating urinary tract function with progressive renal failure was the leading cause of death for persons with chronic SCI [Citation1].
Gradually, the establishment of specialized centres for acute care and follow-up, the introduction of clean intermittent catheterisation (CIC), development of antibiotic and bladder relaxant treatments and an increasing understanding of the nervous control of the lower urinary tract have all contributed to change that situation [Citation2].
Patients with SCI who have access to centralized care now have a lifespan near that of the general population and the main causes of death are cardio-vascular and metabolic [Citation3].
Neurogenic bladder dysfunction in SCI patients is no longer a cause of death in high-income countries, but still represents a sizable morbidity and has a great impact on quality of daily life [Citation4].
Studies on urinary tract complications in chronic SCI were traditionally often limited by selection bias. In general, patients with a higher neurological level and severity of injury and those with complications requiring treatment in tertiary centres have been studied, while there are fewer reports on other samples of patients [Citation5,Citation6]. We report on a regional prevalence group of persons with SCI, followed at a specialized out-patient service, with all neurological levels and severity of injury represented.
The greater Stockholm area has a population of 2.3 million and a prevalence group of around 550 patients with chronic traumatic SCI.
The Stockholm Spinal Cord Injury Study [Citation7], presented in 1996, identified frequent medical problems in this group, among them urinary tract complications such as infections, stone formation, incontinence and kidney dysfunction, and a shortened lifespan compared to the general population. The study also coincided with the start of the Spinalis clinic, a regional outpatient service and follow-up centre for this patient group.
Services at the Spinalis clinic include yearly follow-up visits offered to all patients, focussing on medical complications of SCI. A consultant neuro-urologist was recruited to the clinic at an early stage and has seen patients on a weekly basis since 1995, as well as taken care of diagnostic and surgical interventions.
A comprehensive follow-up programme for neurogenic bladder dysfunction secondary to SCI was established in 2003, and revised in 2013 [Citation8]. It is designed for systematic assessment of all patients with the two-fold responsibility of preserving kidney function and treating urological dysfunctions.
The Stockholm Spinal Cord Uro Study will present our follow-up programme and methods and, based on these, a cross-sectional descriptive survey of the regional prevalence population regarding subjective function as well as objective findings.
Materials and methods
Participants
Participants in this study were men and women aged 18 years or older with a post-traumatic SCI for at least 1 year.
All individuals were living in the greater Stockholm area and registered at the Spinalis clinic, which oversees follow-up for ∼ 90% of the regional SCI population. Four hundred and fifty-three patients were offered participation in the study as they consecutively attended annual check-up visits. Of these, 412 patients consented to participate, constituting 91% of patients who attended the yearly check-up and 75% of the total prevalence group. Fifteen patients declined participation and 26 were excluded.
Reasons for exclusion were old age and multiple other illnesses (n = 10), language difficulties (n = 1), non-traumatic SCI and no residual symptoms (n = 15).
The groups of included vs not included patients were similar in distribution of age, neurological level of injury and severity of spinal cord lesion, as shown in .
Table 1. Study patients and patients not included (excluded and non-attendees).
Table 2. Study patients: neurological level of injury vs AIS grade A–D.
Forty-seven patients did not attend the annual check-up due to the following reasons: current hospitalization/illness (n = 1), chose not to attend (n = 17), unknown reason (n = 33).
Methods
In this study, we attempted to fully apply two programmes for follow-up of chronic SCI: a national Swedish programme for follow-up of medical SCI complications [Citation9], and a regional programme for neurogenic bladder dysfunction [Citation8], complemented with a study-specific questionnaire regarding urinary tract function and complications during the preceding year (Supplementary Appendix 1).
Objective measurements and patient reported data were collected for each individual, as shown in and .
Table 3. Data collection.
Table 4. Interpretation of data and reference values.
Follow-up programme of neurogenic bladder dysfunction post-SCI
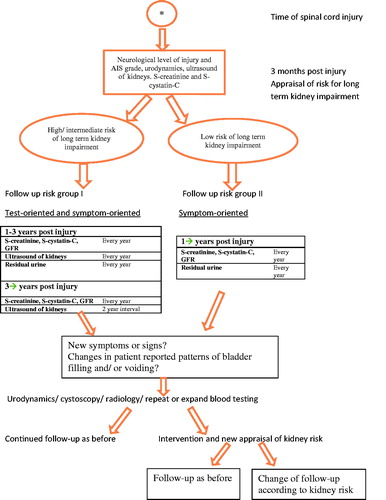
The regional follow-up programme includes patients with all neurological levels and AIS grades of injury (American Spinal Injury Association (ASIA) Impairment Scale) [Citation11] and specifies a system for functional follow-up as well as early detection of kidney deterioration.
The risk of impact on renal function is initially assessed on the basis of neurological level of injury and AIS grade, urodynamic testing, baseline blood tests and renal ultrasound. It is then followed up and re-assessed at specified intervals, as shown in .
Figure 1. Regional follow-up programme of neurogenic bladder dysfunction following spinal cord injury.
Data analyses
Data were analysed using SPSS for Windows 25 software (IBM SPSS Statistics for Windows, Version 25.0. IBM Corp., Armonk, NY). Frequencies and descriptive statistics were calculated. Age, gender, level of injury grouping (C1–C8, T1–T12, Ll–L5, S1–S4), and AIS grades A, B, C, and D, were used as independent variables, as well as types of bladder management, amount of residual urine and urodynamic diagnosis of neurogenic bladder dysfunction. Logistic regression was used to estimate risk factors for kidney impairment.
Results
All patients completed blood tests and the study-specific questionnaire. The number of patients who completed the different steps of the study is shown in . One hundred and ninety-five patients completed all parts of the study.
Objective data
Objective data are summarised in . One hundred and two patients (25%) demonstrated at least one sign of renal complications in blood chemistry and/or ultrasound of the upper urinary tract. Sixty-three patients (15%) had pathological levels of S-cystatin-C and S-creatinine and 56 patients (14%) had abnormal ultrasound findings. Twenty-one patients (5%) demonstrated pathological results in both blood chemistry and ultrasound.
Table 5. Objective data.
All patients with signs of renal complications had cervical or high thoracic neurological lesions and AIS grades A or B. Their SCI was generally of long duration with a mean of 19.7 years and only 10 patients had a duration of less than 5 years. The mean age was 54.8 years, somewhat higher than that of the entire study group. Logistic regression verified the main risk profiles as cervical lesions with AIS grades A–B (odds ratio (OR) = 4.3 vs lumbar-sacral lesions grade D), age (OR = 1.03) and duration of injury (OR = 1.03). There was no difference between bladder management sub-groups.
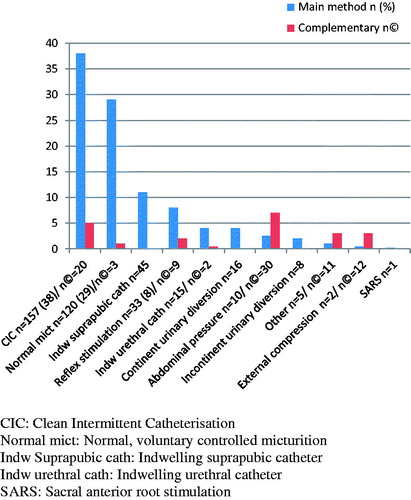
Measurements of residual urine and urodynamic findings were not available for all patients and were therefore evaluated regarding the co-existence with signs of renal complications by descriptive statistics only. In the four most common types of bladder management reported by the patients, those who used normal micturition or reflex stimulation had the highest frequency of residual volume >100 mL (17% and 67%, respectively). Users of clean intermittent catheterisation (CIC) or indwelling catheter had the lowest rates (0% and 6%, respectively).
Patients with residual urine >100 ml (n = 47) had signs of renal complications in only 4%. However, one fourth of patients with pathological kidney findings did not have their residual urine measured.
In the urodynamic studies, patients with all neurological levels and AIS grades of injury were represented, as well as all types of patient reported bladder management ().
The most common finding was neurogenic overactive bladder (nOAB) in 150 patients. Among patients with nOAB, 20% had ≥1 sign of renal complications. However, they were not clearly related to filling pressures. Two thirds demonstrated intravesical filling pressures of >40 cm H2O, a traditionally used reference level for an increased risk of long-term effects on renal function [Citation14,Citation15], but among them only single cases had dilatation of the upper urinary tracts or pathological blood chemistry findings.
Neurogenic underactive bladder (nUAB) was found in 43 patients and a normal detrusor reaction during the filling phase in 18 patients. Notably, one fourth of patients with neurological injury level T1–T12 had nUAB. These patients had in general a longer duration of injury with a mean of 20.3 years/median 22 years.
Patient-reported data from questionnaire and structured interview
All patients completed the questionnaire and structured interview. Bladder management as reported by the patients is shown in . Among patients who use normal voiding, 88% had an AIS grade D and 8% an AIS grade C lesion.
Two hundred and seventeen patients (53%) reported no complications during the preceding year. One hundred and ninety-five patients (47%) had experienced at least one episode of urogenital problems.
Urinary tract infection (UTI) was reported as infection related symptoms which had been treated with a course of antibiotics. This was the most common complication, experienced by 183 patients (44%). The patient reported number of UTIs and other urinary tract complications is summarised in .
Table 6. Patient reported complications during the preceding year.
Risk factors for more frequent or more severe UTIs were identified as high neurological level of lesion and AIS grades A–B, and the use of catheter-assisted voiding.
Sixty-one patients (15%) reported >3 UTIs during the preceding year; 88% had cervical or thoracic neurological level injuries. The highest incidence was found in groups who use CIC (24.5%) and indwelling catheters (20%), as compared with normal voiding (1.7%).
Twenty-one patients (5%) had been treated in hospital due to a febrile UTI. They were all found in groups who practice catheter-assisted micturition, mostly CIC or indwelling suprapubic catheter. Nearly all had a cervical or thoracic neurological level of injury, and AIS grades A and B were present in two-thirds of the group.
Ongoing use of antibiotics or antiseptics to prevent UTI was reported by 115 patients (28%). One hundred and fifty-six patients (38%) used other preventive measures, such as vitamins and health foods. Seventy-five per cent of patients in the antibiotic/antiseptic group and 37% in the non-pharmacological group reported ‘good’ or ‘fair’ prophylactic efficacy. However, the self-reported number of UTIs during the preceding year was not different in these groups compared to the entire study group.
Nearly one quarter of all patients (23%) reported continuous use of bladder relaxant treatment such as anticholinergic medication (70 patients) and botulinumtoxin A (23 patients). Overall satisfaction with these treatments was high, with 92% stating ‘good’ or ‘fair’ efficacy.
Discussion
In the present study, a large proportion (85%) of the regional prevalence group attended the yearly check-up visit, and 91% of them were included in the Stockholm Spinal Cord Uro Study. The findings are, therefore, representative of urological management and secondary complications in the population of persons with chronic post-traumatic SCI in urban Sweden. Our study-specific questionnaire was locally designed as a development of the bladder-kidney section of the national Swedish follow-up programme for SCI complications. There is a good conformity between the types of data recorded in the study-specific questionnaire and in later internationally available questionnaires and data sets, thus facilitating comparison with other studies [Citation16].
The regional programme for bladder dysfunction post-SCI was developed to specify a system for renal and functional follow-up, which would be applicable to all patients registered at the Spinalis clinic. An important aim was to make the programme patient friendly, easy to use, and at the same time accurately informative for medical evaluation. The programme is based on renal ultrasound, S-creatinine and S-cystatin-C in combination, measurement of residual urine, and patient reported functional outcome measures. S-creatinine has well-known limitations in this patient group due to its dependence on muscle mass [Citation17], whereas S-cystatin-C has been shown to give a better representation of glomerular filtration with high sensitivity and specificity in patients with SCI and in some groups with chronic kidney disease [Citation18–20]. In clinical cases of uncertainty regarding the blood chemistry outcome, a nephrologist is consulted. Iohexol clearance is sometimes used as a standard reference on recommendation by the nephrologist.
In the present study, we found that pathological levels of S-cystatin-C were present in all cases with abnormal values of S-creatinine or pathological ultrasound findings, and also in some cases where S-creatinine or ultrasound appeared normal, verifying that S-cystatin-C is a sensitive marker for early kidney affection in patients with chronic SCI.
The results indicate that the majority of our patients are a relatively healthy group with regard to renal function and bladder management, as shown by objective data. Signs of kidney dysfunction and reports on recurring UTIs were present in sub-groups.
Bacteriuria was common, but symptomatic bacterial UTI was present in very few patients at the time of the check-up visit. A random sample of urine from persons who practice catheter-assisted voiding will often show asymptomatic bacteriuria [Citation7,Citation21]. Our figures of symptomatic UTI on random check-up are to our knowledge among the lowest reported so far.
Urodynamics revealed patterns of neurogenic bladder dysfunction which in the majority of cases were concordant with the neurological level of injury. As expected, more than two-thirds had nOAB. However, one quarter of patients with a thoracic neurological injury level had nUAB and at the same time a generally long duration of SCI. It is possible that this group includes a fair number of patients with overdistended or decompensated bladders or possibly post-traumatic descending syringomyelia.
The patients’ subjective data also imply an overall stable situation. A majority of the patients reported no urinary tract complications during the previous year. Most patients were happy with their present method of voiding, with 90% reporting their method as ‘good’ or ‘fair’.
We believe that these encouraging results are due to the system of yearly check-up visits and the availability of urological consultations. Another factor is the substantial number of patients in our population with AIS grade D lesions, who are able to use normal voiding.
However, sub-groups of patients are worse off than the general population.
Objectively, there were pathological findings in blood tests and/or ultrasound as signs of renal impairment in nearly one-fourth of our study patients and they were more common in high-level injuries, AIS grades A and B and injuries of longer duration. While two-thirds of our patients with urodynamically verified nOAB demonstrated episodes of intravesical filling pressures >40 cm H2O, we found no relationship with signs of impaired kidney function. However, some of these pathological signs may be the result of ‘silent’ complications such as long-term increased intravesical pressures. This would support the findings by Elmelund et al. [Citation22] that it is the duration of high intravesical pressure which determines the relationship to long-term kidney impairment.
In the Stockholm area, chronic kidney disease (CKD) has been estimated at 6% of the general adult population accessing primary healthcare [Citation23]. Our study population clearly demonstrates an over-representation regarding objective signs of kidney impairment. New onset of renal dysfunction in traumatic SCI patients has been reported at 4% during 12 years post-injury [Citation24]. Others have found cumulative rates of moderate and severe renal dysfunction at 58% and 29%, respectively, after 45 years of follow-up, and it seems clear that renal deterioration can occur at any time after injury [Citation25].
Our findings indicate that a focussed follow-up of renal function, even with simple blood tests and ultrasound, can reveal more patients in early stages of kidney dysfunction. In this study, we found no relationship with volumes of residual urine, type of bladder management or the number of reported UTIs during the preceding year.
Persons with paraplegia report increased prevalences of diabetes mellitus, hypertension and dyslipidemia compared with the general population [Citation26], factors which are known to imply an increased risk of CKD. Therefore, it seems reasonable that long-term follow-up and interventions to preserve kidney function should include metabolic control as well as urological measures and a multi-professional approach is warranted.
Subjectively, 181 of our study patients reported symptomatic UTI during the preceding year. One third had >3 UTIs per year and 5% had febrile infections requiring treatment in hospital. The importance of active treatment and prevention of infections should be emphasised in follow-up programmes as means to reduce other long-term urinary tract complications, but also the accompanying symptoms of incontinence, spasticity and autonomic dysreflexia, which may lead to immediate difficulties with daily activities.
Our results bear witness to some favourable improvements for persons with SCI over time. In the 1980s, Ruutu et al. [Citation27] found radiological signs of kidney dysfunction in up to 42% of studied groups and others reported a frequency of up to 33% [Citation4]. In the Stockholm Spinal Cord Injury Study in 1995 [Citation8], 6% of all patients had experienced one or more episodes of renal dysfunction, during a mean of 10 years after injury, as verified in patient files. Due to the cross-sectional nature of this study and the focussed follow-up programme, we found a higher prevalence.
Worldwide, urinary tract complications following SCI are more prevalent and often more severe, particularly in low-income regions [Citation28]. Millions of SCI patients do not have access to specialised healthcare, yearly check-up visits or urological consultations. Our results indicate that a stable and relatively healthy situation is possible, but this demands competence, dedication and financial resources. Initiatives at establishing specialised SCI centres in ‘new’ areas of the world point to gratifying outcomes [Citation29].
Several important challenges in urological care of SCI patients remain: to prevent and treat clinically symptomatic UTIs without creating risks for multiresistant bacteria; to develop bladder management methods less dependent on catheterisation; to control intravesical pressure; to remain watchful of kidney function taking the influence of age, duration of injury and metabolic risk factors into perspective; and to make urological follow-up and interventions more available in low-income regions of the world. To make life-long follow-up feasible and acceptable for patients, less invasive methods should be developed, increased self-reporting may be encouraged, and patients should be actively involved in treatment discussions.
Conclusions
With the aid of a regular follow-up programme, chronic SCI patients can achieve a stable and relatively healthy situation regarding urinary tracts and renal function.
Bacteriuria is common, but UTIs are few at a yearly check-up visit.
UTI is by far the most common complication.
Indicators of renal complications are frequent and patients with higher neurological levels of spinal cord lesion and AIS grades A or B are more at risk.
Study-specific_questionnaire.docx
Download MS Word (188.4 KB)Acknowledgements
This work was supported by grants from the Norrbacka-Eugenia Foundation and Wellspect HealthCare. The authors wish to thank Professor Richard Levi for instrumental help in setting up the study and the staff at the Spinalis clinic for performing interviews, database registration and physical examinations.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Whiteneck GG, Charlifue SW, Frankel HL, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia. 1992;30(9):617–630.
- Strauss DJ, Devivo MJ, Paculdo DR, et al. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87(8):1079–1085.
- Thietje R, Kowald B, Hirschfeld S. What are the causes of death in patients with spinal cord injury today?–a descriptive analysis of 102 cases. Rehabilitation. 2011;50(04):251–254.
- Rabadi MH, Aston C. Evaluate the impact of neurogenic bladder in veterans with traumatic spinal cord injury. J Spinal Cord Med. 2016;39(2):175–179.
- Biering-Sørensen F, Nielans HM, Dørflinger T, et al. Urological situation five years after spinal cord injury. Scand J Urol Nephrol. 1999;33(3):157–161.
- New PW. Secondary conditions in a community sample of people with spinal cord damage. J Spinal Cord Med. 2016;1–6.
- Levi R, Hultling C, Nash MS, et al. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia. 1995;33(6):308–315.
- Uppföljningsprogram för neurogen blåsrubbning vid ryggmärgsskada. Mediatryck, Karolinska University Hospital 2003 (revised 2013).
- Levi R, Ertzgaard P. Quality indicators in spinal cord injury care: a Swedish collaborative project. The Swedish Spinal Cord Injury Council 1998. Scand J Rehabil Med Suppl. 1998;38:1–80.
- Asimakopoulos AD, De Nunzio C, Kocjancic E, et al. Measurement of post-void residual urine. Neurourol Urodynam. 2016;35(1):55–57.
- Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535–546.
- Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21(3):261–274.
- Instructions for blood and urine specimens Karolinska University Laboratory (Provtagningsanvisningar Karolinska Universitetslaboratoriet). http://www.karolinska.se/for-vardgivare/KarolinskaUniversitetslaboratoriet/sok-provtagningsanvisning/
- McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126(2):205–209.
- Schöps TF, Schneider MP, Steffen F, et al. Neurogenic lower urinary tract dysfunction (NLUTD) in patients with spinal cord injury: long-term urodynamic findings. BJU Int. 2015;115(Suppl 6):33–38.
- Biering-Sørensen F, Kennelly M, Kessler TM, et al. International spinal cord injury lower urinary tract function basic data set (version 2.0). Spinal Cord Ser Cases. 2018;4(1):60.
- Elmelund M, Oturai PS, Biering-Sørensen F. Biering-Sørensen F. 50 years follow-up on plasma creatinine levels after spinal cord injury. Spinal Cord. 2014;52(5):368–372.
- Erlandsen EJ, Hansen RM, Randers E, et al. Estimating the glomerular filtration rate using serum cystatin C levels in patients with spinal cord injuries. Spinal Cord. 2012;50(10):778–783.
- Hojs R, Bevc S, Ekart R, et al. Serum cystatin C-based equation compared to serum creatinine-based equations for estimation of glomerular filtration rate in patients with chronic kidney disease. Clin Nephrol. 2008;70(07):10–17.
- Heimbürger O, Bárány P. Choose the right method for estimation of kidney function. The nature of the problem determines which method should be used. Lakartidningen. 2009;106(7):420–421.
- Dedeic-Ljubovic A, Hukic M. Catheter related urinary tract infection in patients suffering from spinal cord injuries. Bosn J Basic Med Sci. 2009;9(1):2–9.
- Elmelund M, Klarskov N, Bagi P, et al. Renal deterioration after spinal cord injury is associated with length of detrusor contractions during cystometry-A study with a median of 41 years follow-up. Neurourol Urodynam. 2017;36(6):1607–1615.
- Gasparini A, Evans M, Coresh J, et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. 2016. Nephrol Dial Transpl. 31(12):2086–2094.
- Welk B, Liu K, Winick-Ng J, et al. Urinary tract infections, urologic surgery, and renal dysfunction in a contemporary cohort of traumatic spinal cord injured patients. Neurourol Urodynam. 2017;36(3):640–647.
- Elmelund M, Oturai PS, Toson B, et al. Forty-five-year follow-up on the renal function after spinal cord injury. Spinal Cord. 2016;54(6):445–451.
- Wahman K, Nash MS, Lewis JE, et al. Increased cardiovascular disease risk in Swedish persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med. 2010;42(3):272–278.
- Ruutu M, Kivisaari A, Lehtonen T. Upper urinary tract changes in patients with spinal cord injury. Clin Radiol. 1984;35(6):491–494.
- Gomelsky A, Lemack GE, Castano Botero JC, et al. Current and future international patterns of care of neurogenic bladder after spinal cord injury. World J Urol. 2018;36(10):1613–1619.
- Löfvenmark I, Wikmar LN, Hasselberg M, et al. Outcomes 2 years after traumatic spinal cord injury in Botswana: a follow-up study. Spinal Cord. 2017;55(3):285–289.