Abstract
Objective: We have previously demonstrated protein expression of the extracellular matrix degrading protein ADAMTS5 in the nuclei of urothelial cells in healthy rats. The proteoglycan versican constitutes one of the main substrates for this protease. In this follow up study we investigated a potential co-localization of versican and ADAMTS5 in the urinary bladder wall.
Material and Methods: The study was conducted with archive material (paraffin embedded bladder tissue from our previous study, i.e., 8 male Sprague-Dawley rats). Protein expression of versican was investigated by immunohistochemistry. Furthermore, the occurrence of versican mRNA was examined by in-situ hybridization.
Results: Positive immunoreactivity for versican was evident in the urothelium but also, weakly, in the detrusor. This expression was localized only in the cytoplasm, leaving the nuclei devoid of reactivity. Interestingly, versican mRNA was only sparsely observed in the urothelial cells.
Conclusions: We found by immunohistochemistry that the substrate for ADAMTS5, versican, was localized in the cytosol of urothelial cells. This demonstrates a difference regarding the expression of ADAMTS5, which was emphasized in the nuclei. This could imply an additional, non-enzymatic, function of ADAMTS5 in the urothelium.
Introduction
The extracellular matrix (ECM) of the organism displays a heterogeneous composition that differs between various locations. It consists of a macromolecular network, as built from a selection of collagens, elastin, fibronectin, laminins, glycoproteins, proteoglycans, and glycosaminoglycans, and may be synthesized and secreted by a variety of cell types [Citation1]. The ECM serves at least two main functions, namely structural support and intercellular communication. Within the organism there is a constant turnover of the ECM as brought about by various proteolytic enzymes of which the aggrecanase, ADAMTS5 (A Disintegrin-like and Metalloproteinase with Thrombospondin-1 motifs), is of particular interest. A main substrate for ADAMTS5 is the extracellular chondroitin sulphate proteoglycan, versican, [Citation2] existing in four different isoforms (V0, V1, V2, and V3) [Citation3].
While the occurrence of ADAMTS5 in man has so far been sparsely investigated, this molecule has been found in several tissues in the adult mouse [Citation4]. Furthermore, we recently demonstrated, by immunohistochemistry, for the first time, the cellular localization of the ADAMTS5 protein in normal mammalian (rat) urinary bladder [Citation5]. In addition, we showed that ADAMTS5 immunoreactivity (IR) was expressed not only in urothelial cell cytosol but also in their nuclei, indicating an interplay also at the transcriptional level. ADAMTS5 is implied to function as a major component in certain physiological and pathophysiological processes, e.g., embryological development and osteoarthritis [Citation6]. Versican has been reported to be abundant in a variety of tissues e.g., the brain [Citation7] and skin [Citation8]. Moreover, marked expression of versican has been observed in fast growing tissues, both normal and cancerous, suggesting a role in cell proliferation, see Sotoodehnejadnematalahi et al. for references [Citation3]. With particular reference to the urinary bladder, there seems to be some conflicting results as to the localization of versican. Thus Hurst et al. reported that in human bladder biopsies, versican IR was found to be expressed exclusively in blood vessels of the bladder wall [Citation9], albeit sparsely. Conversely, Bode-Lesniewska et al. [Citation10] found a clear-cut versican IR in the sub-basal connective tissue of human autopsy bladder material. Furthermore, Ozbilgin et al. [Citation11] recently reported positive IR for versican in murine urinary bladder mucosa.
In order to further elucidate a possible physiological role of versican and ADAMTS5 in the urinary bladder we aimed, with the present study, to investigate a potential co-localization of versican and ADAMTS5 in the tissue compartments of the urinary bladder wall.
Material and method
Tissues
The current study, being a follow-up to our previous one, demonstrating ADAMTS5-immunoreactivitry in rat urinary bladder [Citation5] was conducted with archive material (paraffin embedded bladder tissue from that study, i.e., 8 male Sprague-Dawley rats). For details concerning animal procedures and technical details, consult Delbro et al. [Citation5].
Immunohistochemistry
This investigation was undertaken with the MACH1 Universal HRP-Polymer Detection Kit (Biocare Medical, Concord, CA, USA). All procedures were performed at room temperature unless otherwise specified. After deparaffinization and rehydration, the sections were immersed in 10 mM citrate buffer (pH 6), then placed in a microwave oven at medium power for 2 × 5 min for antigen retrieval. Next, endogenous peroxidase was blocked by Peroxidazed 1 (Biocare Medical) for 5 min. Non-specific protein binding was then blocked by Background Sniper (Biocare Medical) for 15 min. The slides were incubated overnight at 4 °C in a moist chamber with the primary antibody (rabbit monocloncal anti-Versican antibody [Abcam, Cambridge, UK; catalogue nr ab177408]; diluted 1:25-1:100 in Da Vinci Green Diluent; Biocare Medical). According to the Manufacturers, this antibody reacts with the various versican isoforms). The MACH1 Universal HRP–Polymer was then added before incubation for 30 min in a moist chamber. Staining was performed using 3,3′-diaminobenzidine (DAB) solution (Biocare Medical). Positive IR was manifested by brown staining. The sections were then counterstained with Mayer’s haematoxylin (Histolab, Gothenburg, Sweden) and after drying in an oven at 60 °C for 15–20 min, the sections were finally mounted with Pertex (Histolab) and were photographed under a light microscope. Negative controls were established by excluding the primary antibody and incubating the tissues with Da Vinci Green Diluent resulting in no IR.
In situ mRNA hybridization
In situ detection of Versican transcripts was carried out on paraffin‐embedded tissue sections using the RNAScope 2.5 HD assay – RED (Advanced Cell Diagnostics, Inc., Hayward, CA, USA). Sections were pretreated using the standard protocol (Formalin-Fixed Paraffin-Embedded (FFPE) Sample Preparation and Pretreatment) followed by hybridization and detection under normal conditions according to manufacturer’s instructions for the manual assay (RNAScope 2.5 HD Detection Reagent – RED). RNAScope probe for Versican, and standard negative DapB (a bacterial gene) and positive polR2A control probes were used (Advanced Cell Diagnostics, Inc). Slides were counterstained with haematoxylin (Histolab).
Results
Immunohistochemistry
In all specimens investigated, IR for versican was evident, in a concentration dependent manner, mainly in the urothelium but also to a certain degree in the detrusor (negative control] and (B). However, such IR was generally not seen in the lamina propria, except for a very sparse expression in the connective tissue (.
Figure 1. Representative images of the protein expression of versican in rat urinary bladder. (A) The tissue was stained without primary antibody against versican (negative control). (B and C) Application of the anti-versican antibody to the tissue resulted in immunoreactivity in the urothelium (U) and to a far lesser extent also in the detrusor muscle (D) and the lamina propria (Lp). Arrow points at microvilli-like structures at the urothelial surface. Scale bar: 100µm (A, B), 50µm (C).
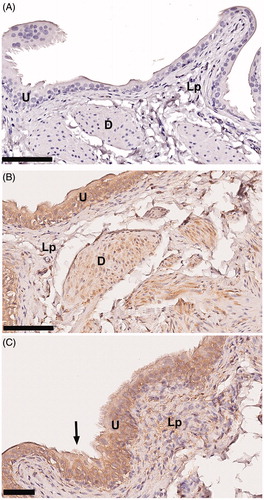
The most superficial urothelial cells expressed microvilli-like structures extending towards the lumen, supporting findings reported in normal human urinary bladder [Citation12] (.
In-situ hybridization
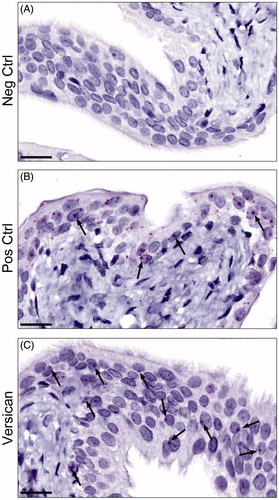
The observation of versican IR in the urothelium was further corroborated by the demonstration, sparse albeit clear, of mRNA for versican in this compartment of the bladder (). The signal of the positive control utilised was however, markedly weaker than the standard one presented in the product sheet from the manufacturers.
Figure 2. Representative images of the mRNA expression of versican in rat urinary bladder visualized using RNAscope® technology. (A) The urinary bladder was stained with DapB as a negative control. As expected no staining was seen. (B) Staining instead with polR2A as a positive control, generated a clear-cut positive reaction in the urothelial cells (arrows) and to a markedly lower extent in the remaining parts of the bladder. (C) The urinary bladder was stained for versican. Sparse, albeit clear, positive staining (arrows) was found in the urothelium, but also in scattered cells throughout the lamina propria, and the muscle cells (not shown in figure). Scale bar: 20 µm.
Discussion
Herein, we demonstrated versican protein expression predominantly in the urothelium but also in the detrusor muscle. These findings were also substantiated by the urothelial localization of versican mRNA. To the very best of our knowledge, this study is the first one to report intracellular expression of versican in (mainly) the urothelial compartment of the healthy mammalian bladder. Our results (found in rats), support those by Ozbilgin et al. (obtained in mice) [Citation11], that versican is expressed in the urothelial compartment. Conversely, Bode-Lesniewska et al. [Citation10] and Hurst et al. [Citation9] described versican IR being restricted to sub-basal connective tissue, and to vascular tissue respectively. The discrepancy between the findings by Bode-Lesniewska et al. [Citation10] and the current study (apart from species differences) may speculatively reflect different antibodies used. Thus, while ours reacts with all four isoforms of versican, that by Bode-Lesniewska et al. only reacts with V0 and V1. If so, our results may suggest that urothelial versican is manifested in the V2 and/or V3 isoforms.
The discrepancy between the findings by Hurst et al. [Citation9] and ours (apart from species differences) may be due to the fact that in the former biopsies were obtained from bladder cancer patients under surveillance and it is conceivable that the urothelium of such patients might be phenotypically unstable.
As mentioned, versican is a proteoglycan with a multitude of functions in normal physiology in relation to its extracellular localization [Citation3]. For example, versican is one of the most abundant type of proteoglycans in the ECM, where it forms a viscoelastic gel embedding collagens and elastic fibers [Citation3]. Proteoglycans are built up by a core protein to which polysaccharide chains, so called glycosaminoglycans (GAG), are covalently attached [Citation13]. Proteoglycans are subdivided into heparan sulphate proteoglycans, chondroitin sulphate proteoglycans and dermatan sulphate proteoglycans, or keratan sulphate proteoglycans depending on the type of GAG chains attached [Citation13].
It is generally agreed upon that chondroitin sulphate proteoglycans, such as versican, play an important role for the barrier between the bladder tissue and the urine; the luminal contents of the bladder may inflict an insult to the underlying tissue compartments in case of barrier defects. For example, it has been proposed that a disruption of the GAG layer might be a contributing mechanism for the development of interstitial cystitis [Citation14,Citation15]. Apart from the extracellular functions of versican, in the current study we noted versican IR predominantly in the intracellular compartment of the urothelium. Intracellular expression of versican has indeed been demonstrated previously [Citation16,Citation17]. Thus in a recent paper Carthy et al. [Citation16] suggested an intracellular function of versican in vascular smooth muscle cells. They speculated that this molecule might play a role in mitotic spindle organization during cell division. Moreover, based upon GAG measurements in blood and plasma, it was suggested that a variability in the expression of various GAG subtypes was associated with progression-free survival in patients with renal cell carcinoma [Citation18]. Our findings, when taken together with previous reports [Citation16–18], open up a novel field to explore, concerning the intracellular role, perhaps even at the transcriptional level, of primarily extracellular molecules. In order to gain further knowledge on the role of versican in this particular respect, experiments are ongoing at our laboratory on versican expression in normal and diseased human bladder.
The findings herein, combined with our recent discovery that ADAMTS5 is expressed intracellularly in the urothelium of healthy rat bladders [Citation5], suggest that there is a co-localization of versican and ADAMTS5 in this tissue. However, the latter molecule was clearly demonstrated also in the nuclei of urothelial cells whereas the former could only be observed in the cytoplasm. Speculatively, ADAMTS5 might, at the transcriptional level, play some other role apart from its enzymatic function as suggested by Kumar et al. [Citation19].
Strengths and limitations
An obvious limitation with the present study is that it is undertaken in rats and, hence, the data obtained herein is not directly transferable neither to human physiology nor pathology. This limitation is, however, also partly a strength. Our modus operandi, using healthy young mammalian tissue, has allowed us to circumvent the interpretive problems arising from immunohistochemical investigation of biopsies from elderly patients with perhaps concomitant pathology and/or age degenerative processes. In our current study, there was a discrepancy between the very sparse expression of versican mRNA and the intensive IR in the urothelium. Explanations for such remain speculative and may include e.g., a higher degree of molecular stability of the versican protein as compared with mRNA, making the demonstration of the protein evident. Similarly, our positive control probe for the in situ hybridization analysis, produced a considerably weaker signal than the one demonstrated in the product sheet. This observation could indicate suboptimal conditions for in situ hybridization using the current tissue.
Conclusion
In this study, undertaken with healthy rat urinary bladder, we found by immunohistochemistry that the chondroitin sulphate proteoglycan versican, similarly to ADAMTS5, was expressed in the urothelium. This seemingly co-localization was restricted to the cytosol of the urothelial cells and, in contrast to ADAMTS5, versican IR could not be shown in the nuclei.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Theocharis AD, Skandalis SS, Gialeli C, et al. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27.
- Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8(10):2975–2981.
- Sotoodehnejadnematalahi F, Burke B. Structure, function and regulation of versican: the most abundant type of proteoglycan in the extracellular matrix. Acta Med Iran. 2013;51(11):740–750.
- McCulloch DR, Le Goff C, Bhatt S, et al. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009;9(5):314–323.
- Delbro D, Hallsberg L, Peeker R, et al. The extracellular matrix-degrading protein ADAMTS5 is expressed in the nuclei of urothelial cells in healthy rats. Scand J Urol. 2018;52(2):139–142.
- Kintakas C, McCulloch DR. Emerging roles for ADAMTS5 during development and disease. Matrix Biol. 2011;30(5–6):311–317.
- Perides G, Rahemtulla F, Lane WS, et al. Isolation of a large aggregating proteoglycan from human brain. J Biol Chem. 1992;267(33):23883–23887.
- Zimmermann DR, Dours-Zimmermann MT, Schubert M, et al. Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J Cell Biol. 1994;124(5):817–825.
- Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology. 2007;69(4):S17–S23.
- Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, et al. Distribution of the large aggregating proteoglycan versican in adult human tissues. J Histochem Cytochem. 1996;44(4):303–312.
- Ozbilgin MK, Aktas C, Uluer ET, et al. Influence of radiation exposure during radiotherapy. Evidence for the increase of versican and Heparin-Binding EGF-like growth factor concentrations. Anal Quant Cytopathol Histopathol. 2016;38:126–132.
- Anderstrom CR, Fall M, Johansson SL. Scanning electron microscopic findings in interstitial cystitis. Brit J Urol. 1989;63:270–275.
- Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6(3):861–870.
- Peeker R, Fall M. Treatment guidelines for classic and non-ulcer interstitial cystitis. Int Urogynecol J. 2000;11(1):23–32.
- Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urology. 1991;145(4):732–735.
- Carthy JM, Abraham T, Meredith AJ, et al. Versican localizes to the nucleus in proliferating mesenchymal cells. Cardiovasc Pathol. 2015;24(6):368–374.
- Hou C, Liu ZX, Tang KL, et al. Developmental changes and regional localization of Dspp, Mepe, Mimecan and Versican in postnatal developing mouse teeth. J Mol Hist. 2012;43(1):9–16.
- Gatto F, Maruzzo M, Magro C, et al. Prognostic value of plasma and urine glycosaminoglycan scores in clear cell renal cell carcinoma. Front Oncol. 2016;6:253.
- Kumar S, Sharghi-Namini S, Rao N, et al. ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. Am J Pathol. 2012;181(3):1056–1068.
