Abstract
Aim: Gonadotropin-releasing hormone (GnRH) agonists are used to treat men with prostate cancer (PCa). To date, no study has fully assessed patterns of adherence to GnRH agonists. We investigated patterns of adherence to GnRH agonists using data from Prostate Cancer data Base Sweden (PCBaSe).
Methods: PCBaSe links the National Prostate Cancer Register (NPCR) Sweden to other healthcare registers and demographic databases. Men on primary or secondary GnRH agonists between 2006–2013 entered the study 45 days after GnRH agonists’ initiation (run-in period) and exited at 3 years. Medication possession ratio quantified adherents (≥80%). Multivariable logistic regression models included age, injection interval, PCa risk categories, Charlson Comorbidity Index, prior PCa treatment, civil status and year of GnRH initiation. Odds ratios (OR) and 95% confidence intervals (CI) expressed odds of adherence.
Results: Men on primary GnRH agonists (n = 8,105) were more adherent with increasing age (75–84 years compared to ≤65 years OR: 1.49; 95% CI: 1.23–1.81), longer injection intervals (365 days compared to 90 days OR: 3.29; 95% CI: 2.52–4.30) and higher PCa risk categories at diagnosis (distant metastasis compared to low risk PCa OR: 3.56; 95% CI: 2.54–5.00). Men on secondary GnRH agonists (n = 4,738) were more adherent with increasing age (≥85 years compared to ≤65 years OR: 1.65; 95% CI: 1.23–2.22) and prior PCa treatment (anti-androgens compared to deferred treatment OR: 1.50; 95% CI: 1.23–1.82), (radiotherapy compared to deferred treatment OR: 1.35; 95% CI: 1.11–1.64).
Conclusions: Longer injection intervals could be addressed in the clinical setting to improve adherence.
Introduction
Androgen deprivation therapy (ADT) is the standard form of treatment for men with advanced prostate cancer (PCa). Considering that around 50% of men diagnosed with PCa may remain on ADT for the rest of their PCa treatment, there is a need to understand factors related to adherence to ADT [Citation1]. No study has fully investigated patterns of adherence to Gonadotropin-releasing hormone (GnRH) agonists, the most common ADT in men with PCa. We assessed this using data from Prostate Cancer data Base Sweden (PCBaSe) [Citation2].
Previous studies in breast cancer have reported side-effects to be a major cause for non-adherence to ADT. Forty-six per cent women who underwent hormonal therapy for breast cancer withdrew from their treatment due to unwanted side-effects associated with the hormonal therapy [Citation3]. Side-effects associated with use of GnRH agonists are related to the castrate levels of androgen and include sexual dysfunction leading to impotence and loss of libido, fatigue, hot flushes, decreased insulin sensitivity, low bone density (leading to increased risk of fractures) [Citation4–7].
Some men with PCa receive intermittent GnRH agonists to minimise the side-effects attributed to GnRH agonists while maintaining anti-tumour efficacy [Citation8,Citation9]. During an intermittent regimen, active treatment periods may be separated by periods without any form of treatment. These active treatment periods by GnRH agonists may last for 6–9 months or until a prostate-specific antigen (PSA) nadir of <4 ng/mL has been reached [Citation10].
It is important to understand the patterns of adherence to GnRH agonists as discussed above. Although some issues surrounding non-adherence to GnRH agonists in men with PCa have been explored previously [Citation11], no studies in the literature have fully investigated patterns of adherence to GnRH agonists for PCa. Our aim was to identify patterns influencing adherence in men with PCa on GnRH agonists over 3 years.
Methods
Study population
PCBaSeTraject version 4.0 [Citation12] links National Prostate Cancer Register of Sweden (NPCR) to other healthcare registries and demographic databases [Citation12,Citation13]. Information on tumour stage, Gleason grading, serum level PSA and primary treatment is registered in the NPCR [Citation14]. PCBaSe links NPCR to registries such as the Swedish Cancer Registry, the Cause of Death Register, the Prescribed Drug Register and the National Patient Register by use of the unique Swedish Personal Identity Number (PIN) [Citation2]. PCBaSe has undergone several extensions with more cases, longer follow-up, family history of PCa and a selection of men free of PCa at the time of sampling (PCBaSe 2.0). PCBaSe 4.0 included men diagnosed with PCa between 1998 and 2016 [Citation12].
During the study period, recommendations for PCa treatment in Sweden were set by regional clinical care guidelines based on national recommendations from the National Board of Health and Welfare. The guidelines stated that once castration by ADT is initiated, it should not be discontinued [Citation15].
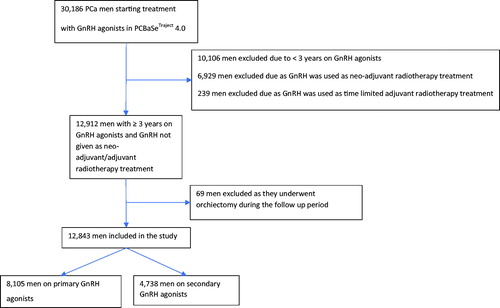
Men with PCa entered the study on date of GnRH agonists’ initiation (plus 45 days) between 2006–2013 and exited at 3 years. A ‘run-in period’ of 45 days was used to avoid overestimating adherence because 90 days (11.25 mg) injection was prescribed most commonly in PCBaSe [Citation16,Citation17]. Primary GnRH agonists were defined as the first form of PCa treatment. Secondary GnRH agonists were defined as men who received other forms of PCa treatments prior to GnRH agonists. Adherence to GnRH agonists was quantified by medication possession ratio (MPR) [Citation18], with a MPR of ≥80% defined as adherent and <80% as non-adherent. Study population was selected using the inclusion and exclusion criteria in .
Statistical analysis
Separate multivariable logistic regression models were conducted for men receiving GnRH agonists as primary and secondary treatment. The odds ratio (OR) expressed the odds of being adherent. Adherence was defined over 3 years following GnRH agonists’ initiation. The multivariable regression models for primary GnRH agonists included: age, injection interval, risk group at diagnosis, change in Charlson Comorbidity Index (CCI) since diagnosis, year of GnRH agonists’ initiation and civil status. Regression models for secondary GnRH agonists included: age, change in CCI since diagnosis, prior PCa treatment and civil status.
Among non-adherent men (<80% MPR), we explored whether the low MPR was due to an intermittent treatment regimen or due to an end in the treatment regimen, in a sensitivity analysis. End to treatment was determined to be a gap of ≥9 months between the last and second last prescriptions [Citation9]. Men without such an end to treatment were considered to be on an intermittent treatment regimen [Citation9]. Once a distinction was made between intermittent GnRH agonist users and those who quit, logistic regression models for primary and secondary GnRH agonists above were conducted using the reclassified outcomes.
We also conducted a sensitivity analysis by redefining adherents as MPR of ≥50% and non-adherents as MPR of <50%, to account for evidence to suggest that testosterone levels may remain suppressed for a longer period of time after treatment with GnRH agonists than previously thought [Citation19].
For men who were 65 years old or younger, we conducted a sub-group analysis to identify patterns of adherence to GnRH agonists specific to this age-group of PCa population.
All analyses were conducted using Software for Statistics and Data Science (STATA) version 15.
Results
8,105 men with PCa starting on primary GnRH agonists and 4,738 men with PCa starting on secondary GnRH agonists between 2006 and 2013 were extracted from PCBaSeTraject (). 79% on primary and 71% on secondary GnRH agonists were adherent after 3 years. Mean age was similar for primary (adherent = 77, standard deviation (SD)=7.8; non-adherent = 76, SD = 8.4) and secondary (adherent = 76, SD = 7.8; non-adherent = 75, SD = 8.0) GnRH agonists.
Table 1. Characteristics for PCa men on primary and secondary GnRH agonists after 3 years.
Primary GnRH agonists
outlines the results of a logistic regression on primary GnRH agonists. Increased adherence was observed in the age-groups 66–74 (OR: 1.27; 95% CI: 1.04–1.54) and 75–84 (OR: 1.49; 95% CI: 1.23–1.81) compared to ≤65 years. Men with PCa on the 365 days injection implant were three times more likely to be adherent than men on 90 days injection interval (OR: 3.29; 95% CI: 2.52–4.30). Men given a dose with 180 days interval between injections were nearly three times more likely to be adherent than those given a dose with 90 days interval between injections (OR: 2.60; 95% CI: 1.89–3.58, Table A6, Supplementary Appendix). Men with distant metastatic PCa were more likely to be adherent than men with low risk PCa (OR: 3.56; 95% CI: 2.54–5.00). A longer follow-up of 6 years showed increased adherence with increased age, increased injection interval and higher risk PCa groups (Table A4, Supplementary Appendix).
Table 2. Logistic regression analyses showing odds ratios (OR) and 95% confidence intervals (CI) for PCa men after 3 years on primary GnRH agonists.
Secondary GnRH agonists
shows the results of a logistic regression for secondary GnRH agonists. Increased age was associated with increased adherence in men who were given GnRH agonists as a secondary treatment for their PCa, with the most adherence observed in men aged ≥ 85 years (OR: 1.65; 95% CI: 1.23–2.22). An increased adherence was also observed with men given longer injection intervals (365 days injection interval OR: 2.65; 95% CI: 2.00–3.51) compared to men given GnRH agonists at 90 days intervals. An increased adherence was observed in men who were given anti-androgens (OR: 1.50; 95% CI: 1.23–1.82) and radiotherapy (OR: 1.35; 95% CI: 1.11–1.64) as primary treatment prior to GnRH agonists’ initiation compared to deferred treatment. Men who were given radiotherapy ≥1 year after undergoing radical prostatectomy were also more likely to be adherent to secondary GnRH agonists compared to no radiotherapy (OR: 1.54; 95% CI: 1.04–2.28).
Table 3. Logistic regression analyses showing odds ratios (OR) and 95% confidence intervals (CI) for PCa men after 3 years on secondary GnRH agonists.
Sensitivity analyses
After 6 years on GnRH agonists, in comparison to deferred treatment, increased adherence was observed in men who were given anti-androgens (OR: 1.67; 95% CI: 1.22–2.29) as prior PCa treatment, whereas decreased adherence was observed in men who underwent radiotherapy (OR: 0.73; 95% CI: 0.56–0.97) (Table A5, Supplementary Appendix).
Following reclassification based on intermittent GnRH agonists therapy, 89% (7,227/8,105) men with PCa on primary GnRH agonists were adherent and 11% (878/8,105) were non-adherent. 86% (4,049/4,738) men with PCa on secondary GnRH agonists were adherent and 15% (689/4,738) were non-adherent. Table A1, Supplementary Appendix shows ORs and 95% CIs estimated using logistic regression models on the reclassified outcomes. Increased age, longer injection intervals and higher risk groups showed an increased adherence in men on primary GnRH agonists. Reclassification of outcomes in the primary GnRH agonists’ group showed that change in CCI by 3 compared to no change in CCI (OR: 1.95; 95% CI: 1.15–3.33) was also statistically significant which was not observed in the original analysis. For the men on secondary GnRH agonists (Table A2, Supplementary Appendix), similar patterns as the original analysis were observed with age, injection intervals and prior PCa treatments (anti-androgens, radiotherapy and radiotherapy ≥1 year after radical prostatectomy) affecting adherence patterns.
Following redefinition of outcomes, a MPR of ≥50% was considered as adherent and a MPR of <50% was considered as non-adherent to GnRH agonists. 88% (7,140/8,105) men on primary GnRH agonists were adherent and 12% (965/8,105) were non-adherent. 84% (3,959/4,738) men on secondary GnRH agonists were adherent and 16% (779/4,738) were non-adherent. Increased age, longer injection interval and higher risk groups showed an increased adherence in men on primary GnRH agonists (Table A7, Supplementary Appendix). For men on secondary GnRH agonists (Table A8, Supplementary Appendix), increased adherence was observed with increased age, longer injection intervals and those who were given anti-androgens or radiotherapy as PCa treatment before GnRH agonists.
Following sub-group analysis including only men aged ≤65 years after 3 years, 76% (612/802) men on primary GnRH agonists were adherent and 24% (190/802) were non-adherent. 65% (348/533) men on secondary GnRH agonists were adherent and 35% (185/533) were non-adherent. No remarkable differences in patterns of adherence to GnRH agonists were observed in men receiving primary (Table A9, Supplementary Appendix) and secondary (Table A10, Supplementary Appendix) GnRH agonists.
Discussion
This is the first nationwide population-based register study to investigate patterns of adherence to GnRH agonists in men with PCa. Increased adherence to primary GnRH agonists was observed with increased age, a longer injection interval and a diagnosis of high risk or metastatic PCa after 3 years. Adherence to secondary GnRH agonists was stronger with increased age, longer injection intervals and prior use of anti-androgens and radiotherapy. Reclassification and redefinition of outcomes showed similar patterns as above and no remarkable differences in associations were observed with a longer study period of 6 years.
An increased age was associated with increased adherence to GnRH agonists for both primary and secondary GnRH agonists. Several studies [Citation20,Citation21] on heart failure medication adherence have shown age to be a determinant of medication adherence. Older individuals with chronic illnesses were more likely to be adherent to their medication than their younger counterparts. Sub-group analysis for men aged ≤65 years was conducted as side-effects from GnRH agonists such as loss of libido may be more of a concern for men aged 65 years or younger. However, no remarkable differences for patterns of adherence to GnRH agonists were observed in this sub-population of men with PCa in our study (Tables A9 and A10, Supplementary Appendix). This requires further investigation as the sample size used in this study was small for this sub-population which resulted in wide CIs.
Men with PCa receiving GnRH agonists with 365 days (50 mg) interval in between injections showed an increased adherence as compared to men receiving the injection with 90 days interval. This may be due to the reduced number of visits required to administer the higher dose injections which means that men on the longer injection intervals may simply be more receptive to the less frequent and more convenient injection schedules [Citation22]. Men treated with the 365 days injection interval showed three times more adherence than those on 90 days injection interval. This warrants further discussion among clinicians into 365 days implants to be offered as an alternative to men encountering difficulties organising appointments at set intervals for injection administration.
Men with metastatic PCa at diagnosis were three times more likely to be adherent to GnRH agonists than men diagnosed with low risk PCa. Since increased disease severity is usually associated with more severe symptoms, men with metastatic PCa may be more likely to adhere to their cancer treatment in order to relieve disease symptoms such as bone pain [Citation23]. However, stage-specific treatment guidelines in Sweden may have predominantly influenced the results of our study. Some men with low-risk PCa may be on GnRH agonists with an elective intent (i.e. men with low-risk PCa may be given treatment instead of no treatment) leading to the low adherence observed this group [Citation24]. Although guidelines [Citation24] in Sweden suggested the use of oestrogens for metastatic PCa because of similar effects to GnRH agonists at a lower cost, our study did not account for oestrogens as it was extremely uncommon in the dataset.
Men who had received radiotherapy prior to GnRH agonists’ initiation were more likely to be adherent to GnRH agonists than those who were on deferred treatment. In men who had undergone radiotherapy for PCa, having radiotherapy ≥1 year after their radical prostatectomy improved adherence to GnRH agonists which may reflect the treatment regimen for an advanced or recurrent PCa. Recommended therapies for localised PCa in Sweden include: radical prostatectomy, radiation therapy [Citation25], anti-androgen monotherapy [Citation26] or a combination of any of these based on cancer risk category and life expectancy. GnRH agonists can be given after a radical prostatectomy to reduce the risk of recurrence and to men who have a PSA relapse. Once PSA is under control, physicians can decide to discontinue GnRH agonists in some cases [Citation27]. Differences in the radiotherapy regimens between localised and advanced or recurrent PCa, therefore, explain the adherence patterns discussed above.
Men given anti-androgens prior to their GnRH agonists were also more adherent than those on deferred treatment. Although some men can continue anti-androgens in combination with GnRH agonists (for one month or longer) because it can help relieve the side-effects caused by GnRH agonists [Citation28], further research is required to understand how patterns of adherence to GnRH agonists is related to different anti-androgen regimens in men with PCa.
Reclassifying outcomes showed no remarkable differences to adherence patterns suggesting that adherence in men on primary or secondary GnRH agonists was not affected by whether they were on intermittent therapy. Men on GnRH agonists may be placed on an intermittent treatment regimen in order to minimise the side-effects attributed to GnRH agonists while maintaining anti-tumour efficacy [Citation8,Citation9]. Although this did not seem to influence the results of our study, the lack of a standard definition for intermittent therapy for men on GnRH agonists means that the 9 months gap explored in this study warrants further research.
Redefining adherence to a MPR of 50% cut-off (Tables A7 and A8, Supplementary Appendix) showed no remarkable differences compared to the original analysis ( and ). We redefined outcomes from a MPR cut-off of 80% to a MPR cut-off of 50% in a sensitivity analysis to consider the longer-lasting effects of GnRH agonists. According to Pettersson et al., the 3-month Buserelin implant have substantially longer duration of testosterone suppression than previously documented [Citation19]. Therefore, it was important to investigate this possibility even though we assessed other GnRH agonists in addition to Buserelin.
We did not explore the possibility of a switch in treatment regimens from GnRH agonists to other forms of ADT since very few men switched in the dataset. The study population in PCBaSe was limited to a single country, with limited ethnic diversity, thus reducing the generalisability of the results globally. However, treatment with GnRH agonists may not differ significantly among men with PCa globally. Future research assessing predictive factors once men stop adhering to the treatment may also offer explanations to the patterns observed in PCBaSe. Patient-related factors were not explored in our study because this was beyond the information available in PCBaSe. Patient-related factors such as forgetfulness, side-effects of GnRH agonists and ‘white-coat compliance’ may also contribute to the adherence patterns in men on GnRH agonists [Citation29]. A qualitative study exploring the reasons contributing to non-adherence to GnRH agonists, both from a patient’s and clinician’s perspective may be beneficial to understanding overall adherence in men with PCa on long-term GnRH agonists.
Conclusions
Men with PCa had high rates of adherence to GnRH agonists primarily owing to the method of medication administration by a healthcare professional. Our study identified increased age, advanced cancer stage at diagnosis, longer injection intervals and prior PCa treatment as patterns contributing to increased adherence. The patterns observed in this study provides evidence for some common factors already known among clinicians that can contribute to adherence in men on GnRH agonists. Further evidence-based discussions among clinicians and research on data from other countries are needed to reinforce whether men with low adherence to GnRH agonists can benefit from tailored adjustment to some of the identified factors in the clinical setting to improve adherence.
GnRHAdhe.PCBaSeSupplementTables_SCANJUROL__Revised.docx
Download MS Word (67.4 KB)Acknowledgements
This project was made possible by the continuous work of the NPCR steering group: P Stattin (chair), I Franck Lissbrant (deputy chair), K Hellström (coordinator), F Sandin, M Nyberg, A Widmark, O Bratt, C Thellenberg Karlsson, O Andrén, M Törnblom, S Carlsson, M Hjälm Eriksson, D Robinson, M Andén, J Hugosson, G Ahlgren O Ståhl, O Akre, P Fransson, E Johansson and C Waller, G Malmberg (patient representatives). The authors would also like to thank all the healthcare providers and patients contributing information to NPCR.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bourke L, Kirkbride P, Hooper R, et al. Endocrine therapy in prostate cancer: time for reappraisal of risks, benefits and cost-effectiveness? Br J Cancer. 2013;108(1):9–13.
- Hagel E, Garmo H, Bill-Axelson A, et al. PCBaSe Sweden: a register-based resource for prostate cancer research. Scand J Urol Nephrol. 2009;43(5):342–349.
- Kuba S, Ishida M, Nakamura Y, et al. Persistence and discontinuation of adjuvant endocrine therapy in women with breast cancer. Breast Cancer. 2016;23(1):128–133.
- Dhanapal V, Reeves DJ. Bone health management in prostate cancer patients receiving androgen deprivation therapy. J Oncol Pharm Pract. 2012;18(1):84–90.
- Chang JI, Bucci J. Unusual side effect from a luteinizing hormone-releasing hormone agonist, leuprorelin, in the treatment of prostate cancer: a case report. J Med Case Rep. 2016;10(1):323.
- Derweesh IH, Diblasio CJ, Kincade MC, et al. Risk of new-onset diabetes mellitus and worsening glycaemic variables for established diabetes in men undergoing androgen-deprivation therapy for prostate cancer. BJU Int. 2007;100(5):1060–1065.
- Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52(3):154–179.
- Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181(5):1998–2006.
- Gomella LG, Singh J, Lallas C, et al. Hormone therapy in the management of prostate cancer: evidence-based approaches. Ther Adv Urol. 2010;2(4):171–181.
- Klotz L, O'Callaghan CJ, Ding K, et al. A phase III randomized trial comparing intermittent versus continuous androgen suppression for patients with PSA progression after radical therapy: NCIC CTG PR.7/SWOG JPR.7/CTSU JPR.7/UK Intercontinental Trial CRUKE/01/013. JCO. 2011;29(7_suppl):3–3.
- Santoleri F, Sorice P, Lasala R. Leuprorelin and Triptorelin in the treatment of Prostate Cancer: Medication adherence, persistence and economic evaluation in five years of analysis. Int J Pharm Sci Res. 2014;1(1):101.
- Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort Profile Update: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base—a refined prostate cancer trajectory. Int J Epidemiol. 2016;45(1):73–82.
- Cazzaniga W, Ventimiglia E, Alfano M, et al. Mini review on the use of clinical cancer registers for prostate cancer: the National Prostate Cancer Register (NPCR) of Sweden. Front Med. 2019;6:51.
- Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort Profile: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42(4):956–967.
- Samverkan RCI. Regarding care programs prostate cancer. 2018. [cited 2019 Apr 17]; Available from: https://www.cancercentrum.se/samverkan/cancerdiagnoser/prostata/vardprogram/gallande-vardprogram-prostatacancer/1.-sammanfattning/.
- Hallas J, Gaist D, Bjerrum L. The waiting time distribution as a graphical approach to epidemiologic measures of drug utilization. Epidemiology. 1997;8(6):666–670.
- Grundmark B, Garmo H, Zethelius B, et al. Anti-androgen prescribing patterns, patient treatment adherence and influencing factors; results from the nationwide PCBaSe Sweden. Eur J Clin Pharmacol. 2012;68(12):1619–1630.
- Kozma CM, Dickson M, Phillips AL, et al. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013;7:509–516.
- Pettersson B, Varenhorst E, Petas A, et al. Duration of testosterone suppression after a 9.45 mg implant of the GnRH-analogue buserelin in patients with localised carcinoma of the prostate a 12-month follow-up study. Eur Urol. 2006;50(3):483–489.
- Dunlay SM, Eveleth JM, Shah ND, et al. Medication adherence among community-dwelling patients with heart failure. Mayo Clin Proc. 2011;86(4):273–281.
- Ambardekar AV, Fonarow GC, Hernandez AF, et al. Characteristics and in-hospital outcomes for nonadherent patients with heart failure: Findings from Get With The Guidelines-Heart Failure (GWTG-HF). Am Heart J. 2009;158(4):644–652.
- Crawford ED, Phillips JM. Six-month gonadotropin releasing hormone (GnRH) agonist depots provide efficacy, safety, convenience, and comfort. Cancer Manag Res. 2011;3:201–209.
- Body JJ, von Moos R, Rider A, et al. A real-world study assessing the use of bone-targeted agents and their impact on bone metastases in patients with prostate cancer treated in clinical practice in Europe. J Bone Oncol. 2019;14:100212.
- Nugin H, Folkvaljon Y, Damber JE, et al. Work-up and treatment of prostate cancer before and after publication of the first national guidelines on prostate cancer care in Sweden. Scand J Urol. 2018;52(4):277–284.
- Sandler HM, Mirhadi AJ. Radical radiotherapy for prostate cancer is the 'only way to go'. Oncology. 2009;23(10):840–843.
- Lycken M, Garmo H, Adolfsson J, et al. Patterns of androgen deprivation therapies among men diagnosed with localised prostate cancer: A population-based study. Eur J Cancer. 2014;50(10):1789–1798.
- Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341(24):1781–1788.
- Thompson IM. Flare associated with LHRH-agonist therapy. Rev Urol. 2001;3(Suppl 3):S10–S14.
- Jin J, Sklar GE, Min Sen Oh V, et al. Factors affecting therapeutic compliance: a review from the patient’s perspective. TCRM. 2008;4(1):269–286.