Abstract
Background: There is conflicting evidence about the association between prostate cancer and Lower Urinary Tract Symptoms (LUTS). We aimed to describe the prevalence of LUTS and its association with prostate cancer risk.
Methods: We studied the association between International Prostate Symptom Score (IPSS) and prostate cancer in a population-based sample of men (n = 45,595) aged 50–69 years from the Stockholm3 study. Men with PSA ≥3 ng/ml (n = 4579) underwent systematic prostate biopsies. We used the International Society of Urological Pathology Gleason Grading (ISUP grade) and performed regression analysis for risk of any cancer (n = 1797), ISUP grade ≥2 (n = 840) and advanced cancer, defined as ISUP grade ≥3 or cT ≥3 (n = 353).
Results: 74.6% of all men had no or mild LUTS (IPSS ≤7) and 3.2% had severe LUTS (IPSS >19). Men with any, ISUP grade ≥2 or advanced cancer had lower median IPSS compared to men with benign biopsy (any cancer: 4 (IQR 2–9); ISUP grade ≥2: 4 (2–8); advanced cancer: 4 (2–8); benign biopsy: 6 (3–11); p < 0.05). IPSS was not associated with increased risk of cancer in multivariate analyses (OR (any cancer) 0.97; 95% CI 0.96–0.98; OR (ISUP grade ≥2) 0.97; 95% CI 0.96–0.99; OR (advanced cancer) 0.99; 95% CI 0.99–1.01).
Conclusions: Three-quarters of men aged 50–69 years report no or mild LUTS. Our data do not support any clinically meaningful association between LUTS and prostate cancer. Specifically, men with advanced prostate cancer did not exhibit more urinary symptoms than men without cancer.
Introduction
Lower urinary tract symptoms (LUTS) and benign prostate hyperplasia (BPH) are common urological conditions. Similarly to prostate cancer, the prevalence of LUTS and BPH increases with age [Citation1]. BPH is a known cause of LUTS and often coexist with prostate cancer [Citation2,Citation3]. From a clinical perspective it is of importance to determine whether LUTS also can be indicative of prostate cancer, and whether men with LUTS therefore should undergo diagnostic work up for prostate cancer.
The relationship between prostate cancer, BPH and LUTS has been studied in various settings [Citation4–11]. However, these studies have mostly been conducted on observational data and are prone to ascertainment bias. No consensus has therefore been reached on the specific relationship between LUTS and the risk of cancer. Further, evidence on this relationship in a cohort that is representative for the target population for prostate cancer screening is limited.
Whether to screen for prostate cancer or not is an ongoing debate. With few exceptions, there are currently no formal nationwide screening programs for prostate cancer implemented. However, opportunistic screening is widespread [Citation12]. Today most cancers are diagnosed in asymptomatic men as a result of PSA testing [Citation13,Citation14] even though it was shown that only 1% of the general population was aware that prostate cancer could manifest without symptoms [Citation15]. Still, several national health care bodies including the Swedish national guidelines for management of prostate cancer continue to recommend testing with PSA in patients with urinary symptoms [Citation16]. Further, men with LUTS expect to be tested for prostate cancer. Studies from Australia, where the National Health and Medical Research Council advice against PSA testing on the basis of LUTS, have shown that 75% of patients with LUTS expect to be tested for prostate cancer [Citation17]. Another study showed that 66% of general practitioners would recommend PSA testing in patients with LUTS [Citation18].
We have addressed the questions if it (i) is meaningful to test men with urinary symptoms for prostate cancer in a clinical setting and if it (ii) is relevant to check for LUTS in order to identify men with increased risk of cancer. Our aim was to describe the prevalence of urinary symptoms in a prostate cancer screening population and to investigate whether there is an association between urinary symptoms and the risk of prostate cancer. We also intended to study whether men with advanced cancer were more prone to exhibit LUTS.
Subjects and methods
We used data from the Stockholm3 study [Citation19], a prospective and populations-based prostate cancer diagnostic study in Stockholm, Sweden conducted in 2012–2015. 58,818 randomly invited men aged 50–69 participated in the study. Men with prostate cancer at the time of recruitment were excluded. All participants with PSA ≥3 ng/ml were recommended to undergo prostate biopsies. The number of systematic biopsies was 10 in prostates smaller than 35 ml, and 12 if the volume was equal or greater than 35 ml. Biopsies were performed by urologists and specimens were analyzed by a single, highly experienced uro-pathologist (LE). Both urologists and the pathologist were blinded to PSA levels.
As part of the Stockholm3 study, the International Prostate Symptom Score (IPSS) questionnaire (Supplementary Appendix 1) was sent to all participants.
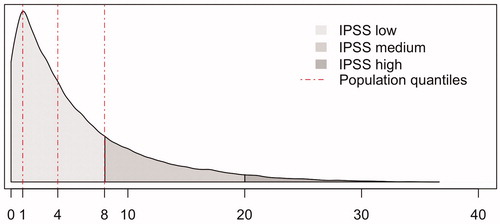
IPSS is a validated and widely used self-reported symptom score calculated from the answers of seven questions regarding urinary symptoms and one regarding quality of life. The urological symptoms evaluated are incomplete emptying, frequency, intermittency, urgency, weak stream, straining and nocturia. The question regarding quality of life was disregarded in this study. Each question renders 0–5 points depending on the severity of the symptom, thus a total of 35 points can be obtained. The total score is categorized as low, medium or high and can then be used to grade LUTS as mild (IPSS 0–7 points), moderate (8–19) or severe (20–35).
We used descriptive statistics to show how urinary symptoms were distributed in the study population and to estimate the prevalence of low, moderate and high LUTS. We used data on the biopsied men with PSA ≥3 ng/ml to study the associations between IPSS and the International Society of Urological Pathology Grade Group (ISUP grade) specific prostate cancer. ISUP grades ranges from 1 (low grade) through 5 (high grade) [Citation20]. We defined advanced prostate cancer as ISUP grade ≥3 or clinical T-stage (cT) ≥3. T-stage was determined by findings on digital rectal examination (DRE). Logistic regression was used to investigate the association between IPSS and prostate cancer. Adjusted analyses included information on age (years), prostate volume (ml), total PSA (log, ng/ml), free PSA (ng/ml), previous biopsy and the use of 5-α-reductase inhibitors (5-ARI). We calculated odds ratio (OR) for ISUP grade specific cancer in relation to IPSS. STATA 14.0 and R version 3.4.3 (StataCorp LLC and R Foundation) were used as software for data management and statistical analysis.
Men who did not respond or had incomplete IPSS questionnaires were excluded from this study. We compared population characteristics between IPSS questionnaire responders and non-responders to test for any differences.
Results
shows characteristics of the study population. The IPSS questionnaire was answered by 45,595 (77.5%) out of 58,818 Stockholm3 participants. The median age of the study population was 60.5 years (55.0–65.5 IQR). The median PSA was 1.1 ng/ml (0.68–2.8 IQR). Men with complete and incomplete information on IPSS had very similar age, PSA-distribution and prostate volumes. The medians differed less than 0.5%.
Table 1. Characteristics of the study population.
The distribution of IPSS in this population-based sample of men aged 50–69 is shown in : 33,995 (74.6%) men had low IPSS (0–7), 10,128 (22.8%) medium IPSS (8–19) and 1472 (3.2%) high IPSS (20–35). More than a third (38%, n = 17,603) had no or very few symptoms (IPSS 0–2) and 25% (n = 11,621) had IPSS 3–5.
Ten percent (n = 4579) of the 45,595 participants with IPSS data had PSA ≥3ng/ml and subsequently underwent prostate biopsies. In this group, 2934 (64.1%) had low IPSS, 1405 (29.2%) medium IPSS and 240 (4.7%) high IPSS. 1797 (39.2%) had a positive biopsy for prostate cancer and 2782 (60.8%) were benign. 840 (46.7%) had ISUP grade ≥2 cancer and 353 (19.3%) had advanced cancer ().
shows the distribution of IPSS in men undergoing biopsies and how IPSS corresponds to risk of cancer. 75% of the studied men registered IPSS ≤10. The risk of any cancer, ISUP grade ≥2 and advanced cancer all decrease with increasing IPSS. No individual symptom included in the IPSS questionnaire was significantly associated with prostate cancer.
Figure 2. The risk of prostate cancer in 4579 men undergoing prostate biopsy subdivided by ISUP grade (colored lines) and the distribution of International Prostate Symptom Score (IPSS; grey). Dotted red line indicates median IPSS.
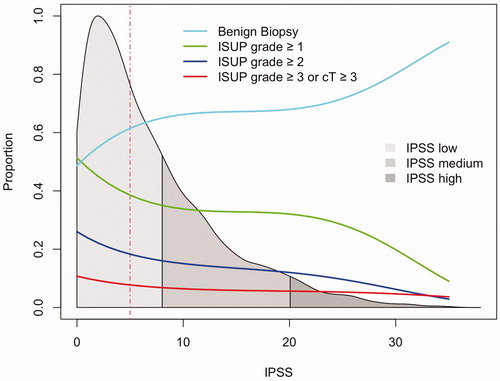
The risk of prostate cancer stratified by level of IPSS is shown in . Less cancer was found in the groups with medium and high IPSS compared to low IPSS; 42% had any cancer in the group with low IPSS, 33% in the group with medium IPSS and 31% in the group with high IPSS. This pattern was even more marked when ISUP grade 1 was excluded: 21% of men with low IPSS were found to have ISUP grade ≥2, compared to 14% and 12% with medium and high IPSS respectively.
Table 2. Risk of prostate cancer stratified by level of IPSS and ISUP grade in men with PSA ≥3 ng/ml.
Participants with any prostate cancer, ISUP grade ≥2 cancer and advanced cancer all had lower median IPSS compared to those with a benign biopsy (any cancer: 4 (IQR 2–9); ISUP grade ≥2 cancer: 4 (2–8); advanced cancer: 4 (2–8); benign biopsy: 6 (3–11); p < 0.05).
Multivariate logistic regression analyses adjusted for age, total PSA, free PSA, T-stage and prostate volume are presented in . Increasing IPSS was not associated with any clinically meaningful influence on risk of cancer. Odds ratios (OR) were 0.97 (0.96–0.98 95% CI) for any cancer, 0.97 (0.96–0.99) for ISUP grade ≥2 and 0.99 (0.99–1.01) for advanced cancer.
Table 3. Multi-variate analysis on the risk of prostate cancer in 4579 men with PSA ≥3 ng/ml undergoing systematic prostate biopsy.
As sensitivity analyses, we performed separate regression analyses including the use of 5-ARI and any previous prostate biopsy procedure. Adjusting for these factors did not significantly change the findings. Tables are included in supplements. The overall use of 5-ARI in the study population was low (1.4%) but there was a considerable difference between men with a positive and negative biopsy for cancer (0.7% and 1.91% respectively). Further, 7.5% of the study subjects had undergone previous prostate biopsies. This was about twice as common in the benign group compared to men with cancer in the biopsy specimen (9.6% and 4.2%).
Discussion
We present population-based and contemporary data that portray the prevalence of LUTS in men 50–69 years, and the association between LUTS and the risk of prostate cancer. Our data shows that three-quarters of men report no or mild LUTS as reported using the validated IPSS questionnaire. Presented analyses do not support any clinically relevant association between LUTS and prostate cancer.
Severe LUTS was not a common problem in the Stockholm3 cohort, which broadly represents the target group for prostate cancer screening. Three out of four men had no or mild symptoms. 22.2% and 3.2% reported moderate and severe LUTS, respectively. Our data are mainly in line with previous studies. In a previous study on Swedish men, Andersson et al. [Citation21] reported that 18.5% had moderate and 4.8% severe LUTS in a cohort of 40,000 Swedish men aged 45–79. In the Multinational Survey of the Aging Male (MSAM-7) data was collected from 12,815 men 50–80 years in Europe and United States, and moderate to severe LUTS were recorded in 31% of all men [Citation22].
Nonetheless, other previous studies present different results. For instance, community data from the UK recorded moderate and severe LUTS in 41% of the population [Citation23]. McVary showed that LUTS increases with age and 50% of men ≥80 years had moderate or severe LUTS [Citation24]. One possible factor contributing to the differences in findings in these and other studies might be the different age-distributions of participants. Another factor to take into consideration is that some studies present prevalence of LUTS in a dichotomous way, simply if it exists or not, and do not specify neither symptoms nor severity of the condition [Citation25].
Our data do not support any clinically meaningful association between urinary symptoms and the risk of harboring prostate cancer. We show that the risk of prostate cancer does not increase with increasing LUTS. On the contrary, we report a slightly lower risk of any, ISUP grade ≥2 as well as advanced prostate cancer (OR 0.97–0.99) with increasing IPSS. The result for advanced cancer was not statistically significant (OR 0.99; 95% CI 0.99–1.01), however, we find no support that men in this group exhibit more LUTS than men without cancer.
A plausible explanation to this negative association is that LUTS is associated with increased prostate volume, which in turn correlate proportionally to increased PSA levels [Citation26]. Following this reasoning, LUTS is associated with lower PSA-specific prostate cancer risk. This supports that verification of IPSS should be omitted from prostate cancer early detection protocols.
Interestingly, the proportion of performed biopsy procedures that showed ISUP grade ≥2 cancer was approximately twice as high in men with low IPSS compared to men with high IPSS (21% vs 12% of biopsy procedures). Proportions of biopsy procedures showing advanced cancer were comparable over IPSS strata. These findings indicate that men with urinary symptoms do not have an increased risk of significant cancer.
Earlier evidence on the association between LUTS and prostate cancer are conflicting, as some studies have argued that there is a positive correlation between LUTS and prostate cancer [Citation4,Citation5], and some that there is not [Citation8,Citation9]. The Prostate Cancer Prevention Trial (PCPT) [Citation27], showed no positive association between BPH and prostate cancer [Citation9]. However, the PCPT used very specific inclusion criteria which make the results with respect to associations between LUTS and prostate cancer less applicable to the general population. Frånlund et al. [Citation10] conducted a study with similarities to ours. They showed that voiding symptoms were negatively correlated to subsequent detection of prostate cancer in prostates larger than 37.8 ml, OR 0.78 (0.63–0.98). However, they did not use a validated symptom score such as IPSS to quantify LUTS and used a sextant biopsy protocol which is no longer standard of care.
Ørsted et al. [Citation5] on the other hand presented a very large observational study of more than 3 million men in Denmark showing that men with LUTS had an increased risk of prostate cancer [HR 2.2 (95% CI: 2.13–2.31)] over a follow-up period of 27 years. A Norwegian study of about 30,000 also showed increased risk of detecting prostate cancer in men with LUTS with HR of 4.6 (2.23–9.54) in men with severe LUTS [Citation11]. One of the main limitations in these studies arguing for a positive association between LUTS and cancer is the ascertainment bias that inevitably occurs in observational studies. Given the association between PSA, prostate volume and urinary symptoms together with the increased testing among men with such symptoms, it is possible that more prostate cancer will be detected in men with urinary symptoms compared to the control groups. Stockholm3 employed a prospective screen-by-invitation design with a pre-defined PSA cutoff for biopsy referrals. Followingly, the problem with ascertainment bias is markedly mitigated.
It is recognized that the growth of adenoma in BPH arises in the transition zone of the prostate, in proximity to the urethra, and therefore is prone to result in LUTS. Cancer on the other hand most often develops in the peripheral zone, hence at some distance from the urethra indicating less likelihood for obstruction of urinary flow [Citation28] especially if lesions are small. Even though we acknowledge other factors in the pathological pathway for prostate cancer, our results in favour of no positive association between LUTS and prostate cancer hereby makes anatomical and etiological sense.
We performed sensitivity analyses to assess the robustness of our results (Supplementary Tables 1 and 2). First, previous benign systematic prostate biopsy is strongly associated with benign findings on subsequent prostate biopsies [Citation19]. This was also the case in our data. Our results did not change when excluding men with a previous prostate biopsy. Secondly, 5-ARI is a common treatment for BPH that might affect the risk of cancer [Citation27]. It reduces the volume of the prostate and alleviates LUTS. In this study we saw that the use of 5-ARI had a very low prevalence (1.4%) but was used almost three times (2.7) as frequent in men without cancer. Although beyond the scope of this paper, these findings are in line with previous studies showing that the use of 5-ARI may reduce the risk of low-grade prostate cancer [Citation27]. Adjusting for 5-ARI did not materially affect the study results. The low prevalence of prostate volume reducing drugs (1.4%) reflects our findings of low frequency of LUTS in our study subjects.
We have several limitations in our study. First, the study population consisted of men 50–69 years whereas incidence of both urinary symptoms and prostate cancer increase in older men. Another limitation is that the biopsied population only included men with PSA ≥3 ng/ml. Thus, extrapolation outside the study populations requires caution. Although the Stockholm3 study represents a large cohort of men and the response rate for IPSS was high (>75%), there could hypothetically be systematic differences in men choosing to answer the IPSS questionnaire compared to those who did not. However, there seemed to be very small differences (<0.5% difference in median) in men with incomplete data compared to men with full data in terms of age, PSA and prostate volume.
Conclusion
Severe LUTS in men aged 50–69 years and PSA ≥3 ng/ml is relatively uncommon. LUTS is not associated with an increased risk of prostate cancer (neither low grade nor advanced cancer). Instead we found that with increased IPSS there is a slightly lower risk of any and ISUP grade ≥2 cancer. Our findings do not support initiation of work up for prostate cancer based on LUTS in a screening setting. Neither do our findings support introduction of LUTS measurements as a tool in early detection of prostate cancer. This should be reflected in current guidelines and followed in clinical practice.
Supplementary_Tables_1_2.docx
Download MS Word (27.1 KB)Appendix_1._IPSS.docx
Download MS Word (5.7 MB)Acknowledgements
The authors thank the Stockholm3 research group at the Department of Medical Epidemiology and Biostatistics, Karolinska Institutet.
Disclosure statement
The funding sources had no role in the collection, analysis and interpretation of data, nor the writing of the report. Martin Eklund has four patents related to prostate cancer diagnostics pending. There are no other potential conflicts of interest for any of the authors.
Additional information
Funding
References
- Speakman M, Kirby R, Doyle S, et al. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int. 2015;115(4):508–519.
- Simon RM, Howard LE, Moreira DM, et al. Does prostate size predict the development of incident lower urinary tract symptoms in men with mild to no current symptoms? Results from the REDUCE Trial. Eur Urol. 2016;69(5):885–891.
- Bostwick DG, Cooner WH, Denis L, et al. The association of benign prostatic hyperplasia and cancer of the prostate. Cancer. 1992;70(1 Suppl):291–301.
- Adolfsson J, Helgason AR, Dickman P, et al. Urinary and bowel symptoms in men with and without prostate cancer: results from an observational study in the Stockholm area. Eur Urol. 1998;33(1):11–16.
- Orsted DD, Bojesen SE, Nielsen SF, et al. Association of clinical benign prostate hyperplasia with prostate cancer incidence and mortality revisited: a nationwide cohort study of 3,009,258 men. Eur Urol. 2011;60(4):691–698.
- Guess HA. Benign prostatic hyperplasia and prostate cancer. Epidemiol Rev. 2001;23(1):152–158.
- Alcaraz A, Hammerer P, Tubaro A, et al. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur Urol. 2009;55(4):864–873.
- Bhindi A, Bhindi B, Kulkarni GS, et al. Modern-day prostate cancer is not meaningfully associated with lower urinary tract symptoms: analysis of a propensity score-matched cohort. CUAJ. 2017;11(1–2):41–46.
- Schenk JM, Kristal AR, Arnold KB, et al. Association of symptomatic benign prostatic hyperplasia and prostate cancer: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173(12):1419–1428.
- Franlund M, Carlsson S, Stranne J, et al. The absence of voiding symptoms in men with a prostate-specific antigen (PSA) concentration of >/=3.0 ng/mL is an independent risk factor for prostate cancer: results from the Gothenburg Randomized Screening Trial. BJU Int. 2012;110(5):638–643.
- Martin RM, Vatten L, Gunnell D, et al. Lower urinary tract symptoms and risk of prostate cancer: the HUNT 2 Cohort, Norway. Int J Cancer. 2008;123(8):1924–1928.
- Nordstrom T, Aly M, Clements MS, et al. Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003-2011. Eur Urol. 2013;63(3):419–425.
- Van Poppel HC, Montorsi F C, et al. Prostate cancer - recommendations to lower the risk and mortality rate of the most frequent cancer in men. European Association of Urology; 2013. https://issuu.com/uroweb/docs/eau_whitepaper_pca_final.
- Cancercentrum R. Regionala Cancercentrum i samverkan. Prostatacancer. Nationell kvalitetsrapport för 2016. 2016. http://npcr.se/wp-content/uploads/2017/10/20170922_npcr_nationell_rapport_2016.pdf
- Schulman CC, Kirby R, Fitzpatrick JM. Awareness of prostate cancer among the general public: findings of an independent international survey. Eur Urol. 2003;44(3):294–302.
- Cancercentrum R. Prostatacancer. Nationellt Vårdprogram. 2018. https://www.cancercentrum.se/globalassets/cancerdiagnoser/prostatacancer/vardprogram/nationellt-vardprogram-prostatacancer.pdf
- Ward J, Girgis S. GPs’ estimates of men’s risk of prostate cancer and screening expectations. Aust N Z J Public Health. 1999;23(2):219–220.
- Young JM, Muscatello DJ, Ward JE. Are men with lower urinary tract symptoms at increased risk of prostate cancer? A systematic review and critique of the available evidence. BJU Int. 2000;85(9):1037–1048.
- Gronberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol. 2015;16(16):1667–1676.
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40(2):244–252.
- Andersson SO, Rashidkhani B, Karlberg L, et al. Prevalence of lower urinary tract symptoms in men aged 45-79 years: a population-based study of 40 000 Swedish men. BJU Int. 2004;94(3):327–331.
- Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol. 2003;44(6):637–649.
- Trueman P, Hood SC, Nayak US, et al. Prevalence of lower urinary tract symptoms and self-reported diagnosed ‘benign prostatic hyperplasia’, and their effect on quality of life in a community-based survey of men in the UK. BJU Int. 1999;83(4):410–415.
- McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122–S128.
- Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–1314. discussion 1314–1315.
- Meigs JB, Barry MJ, Oesterling JE, et al. Interpreting results of prostate-specific antigen testing for early detection of prostate cancer. J Gen Intern Med. 1996;11(9):505–512.
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224.
- McNeal JE, Redwine EA, Freiha FS, et al. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12(12):897–906.