Abstract
Purpose: To determine the rationale for not offering local treatment to prostate cancer patients with non-metastatic disease at diagnosis who later died of prostate cancer and to document local and systemic complications caused by disease progression.
Material and Methods: In this population-based, retrospective study we reviewed the medical records of all patients who died of prostate cancer in 2009–2014 in Vestfold County (Vestfold Mortality Study), who were non-metastatic at diagnosis and who had received no local treatment to the prostate (n = 117).
Results: A review of patient records demonstrated that the chronological age of 75 years or older was the main rationale for not offering local treatment to the prostate (37%, n = 43). No consideration was given to the functional status and patient health. These elderly patients stood for almost one-fifth of the total PC mortality in Vestfold County. In addition to dying from PC, 86% of patients developed local complications attributable to PC progression. Observation of strict limits for local treatment with regard to tumor characteristics contributed further to the underuse of local treatment.
Conclusions: Our study demonstrated systematic undertreatment of elderly patients with aggressive, non-metastatic PC with regard to local treatment based on chronological age alone. The patients in this study died of prostate cancer and the majority experienced significant morbidity caused by local tumor growth.
Introduction
Reducing prostate cancer mortality is the main objective of prostate cancer treatment. Early detection followed by local treatment of the prostate either by surgery or radiation is important means for achieving this goal [Citation1–4].
The Vestfold Mortality Study, which examined the validity of death certificates in determining the cause of death of prostate cancer (PC) patients, identified 255 men who died of PC in Vestfold County, Norway, during the 6-year period 2009–2014 [Citation5]. More than half of these patients (n = 139; 55%) had non-metastatic PC (NMPC) at diagnosis and thus potentially curable disease. Unexpectedly, only 16% of these had been offered local treatment. The median age of patients who had received no local treatment was 75 years suggesting that age might have influenced treatment decisions.
Incidence rates of high-risk and locally advanced PC increase with age [Citation6], while guidelines with regard to local treatment of elderly patients with PC are usually vague. The current EAU guidelines suggest that patients with high-risk, localized PC should have a life expectancy of 10 years or more to be eligible for local treatment while no guidance on patient age is provided for patients with locally advanced PC [Citation7].
The aim of this study was to identify the rationales for offering no local treatment to the prostate in NMPC patients in Vestfold County who later died of PC. Furthermore, we documented systemic and local interventions and complications caused by disease progression.
Material and methods
Patient population
Vestfold Hospital Trust provides PC care for all patients in Vestfold County (Population 240,000, the average annual number of new PC cases 2013–2017: 263) including diagnosis, treatment and follow-up, allowing for access to almost complete patient data. Among the 255 patients who died of PC in 2009–2014 in Vestfold County (Vestfold Mortality Study database) [Citation5], we identified 139 patients with NMPC at diagnosis. A hundred and seventeen (84%) of these patients had not been offered local treatment. Metastatic status was determined by the imaging method available at the time of diagnosis (bone scan, CT, MRI).
Methods
We deducted the rationales for choosing curative versus non-curative treatment from the patient records at the time of diagnosis based on the stated or implied reasoning of the treating doctor. Patients were assigned to two groups according to the decision rationale: Patients who had not received curative treatment due to age (NoTreat/age) and patients who had not received local treatment due to other reasons (NoTreat/other)
We assessed patient performance status retrospectively using the Eastern Cooperative Oncology Group score (ECOG) [Citation8] differentiating patients with good performance status (ECOG 0 and 1) from patients with reduced performance status (ECOG ≥ 2). We recorded patient comorbidity using the Charlson comorbidity index (CCI) and estimated 10-year survival using the age-adjusted Charlson-score (CS) [Citation9].
We collected data on patient survival, tumor characteristics at diagnosis, use of systemic therapy/radiation to bone/prostate and local complications during the course of the disease from patient records.
Statistics
All statistical analyses for the Vestfold patients were conducted using SPSS statistics, version 23 (IBM Corp., Armonk, NY). We described baseline data by the median, interquartile range and percentages.
Results
Rationale for treatment decision
Of the 117 patients with non-metastatic PC at diagnosis who had not received local treatment, 73 (62%) were initially treated with hormones, while 44 patients (38%) were followed with watchful waiting.
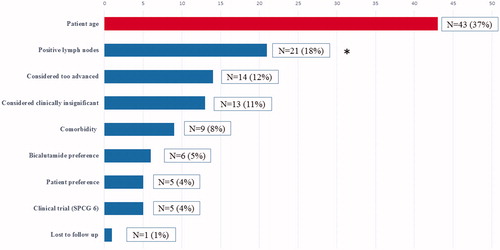
Analysis of the decision process identified chronological age as the reason for abstaining from curative treatment in 37% of patients (NoTreat/age, n = 43). Patients in this subgroup stood for 17% of all PC deaths in Vestfold County during the study period and for more than 30% of PC deaths with NMPC at diagnosis. shows the complete list of rationales for choosing hormonal treatment or watchful waiting for the study population.
Figure 1. Rationales for offering conservative treatment or watchful waiting in non-metastatic patients in the Vestfold Mortality Study (N = 117). *Positive lymph nodes were diagnosed by surgical lymph node staging. According to national treatment protocols, at the time positive lymph nodes were a contraindication for radical treatment.
The median age at diagnosis in the NoTreat/age and NoTreat/other groups was 79 and 70 years respectively, and the median time from diagnosis to death was 7 and 8 years, respectively.
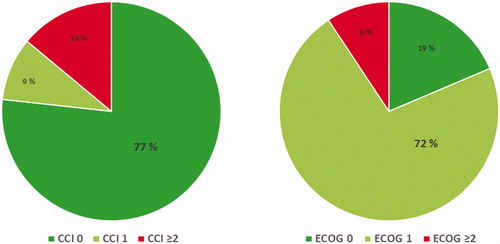
Ninety-one percent of patients in the NoTreat/age group were in good functional shape at the time of diagnosis (ECOG ≤1) and 86% of patients were in excellent or good health (Charlson comorbidity index/CCI ≤1) ().
Figure 2. Charlson Comorbidity Index (CCI, left) and ECOG performance status (right) at diagnosis for patients with non-metastatic prostate cancer who received no local treatment due to age (NoTreat/Age, N = 43).
Almost all NoTreat/age patients had either locally advanced or localized high-risk PC at diagnosis. summarizes the clinical characteristics of both patient groups.
Table 1. Characteristics of all patients with non-metastatic PC at diagnosis, who later died of PC and who received no local treatment (n = 117).
The median year of diagnosis for all patients was 2003 (IQR 2001–2007). Median year of diagnosis for patients in the NoTreat/age group was 2005 while the median year of diagnosis for patients in the NoTreat/other group was 2002.
PC related morbidity
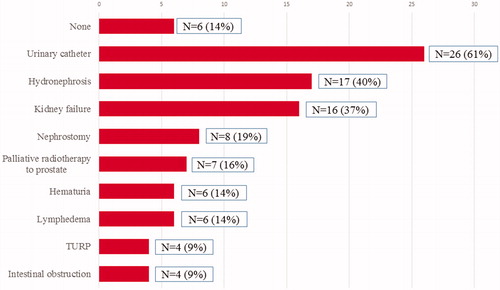
In addition to dying from PC, 86% of patients in the NoTreat/age group developed local complications during the course of their disease. More than half of the patients needed bladder catheterization, 20% a nephrostomy and 10% a colostomy (see ).
Figure 3. Local complications during the course of the disease in patients with no local/curative treatment due to age (NoTreat/Age, N = 43). The total incidence of >100% is a result of the occurrence of two or more complications in a single patient.
Nineteen percent of patients received radiation to the bone (n = 8), 7% were treated with docetaxel alone (n = 3) and 14% were treated with various combinations of palliative therapies. Only one patient (2%) received abiraterone alone and no patient was treated with enzalutamide alone. Almost 60% of patients received no second-line treatment (n = 25).
Forty-seven percent of NoTreat/age patients (n = 20) received blood transfusions at least once due to cancer-induced anemia.
Discussion
In this retrospective, population-based study, we found that many patients with lethal PC but potentially curable disease at diagnosis did not receive local treatment of the prostate based on their chronological age alone (≥75 years). In addition to dying of PC, the majority of these patients experienced considerable morbidity caused by the local progression of PC.
There is increasing awareness that elderly patients with cancer are undertreated, particularly with regard to local, curative therapy [Citation10–12]. For PC globally, the large majority of elderly patients with NMPC has been treated conservatively with hormone therapy or followed with watchful waiting, partly due to a lack of documented benefit of treatment with curative intent [Citation13]. In Norway NMPC in elderly patients was regarded as a condition conferring little risk for patients in terms of mortality and morbidity, regardless of PC grade and stage [Citation14]. In our study, we further documented that in addition to age, stringent national thresholds (PSA, lymph node status) for offering local treatment 10–15 years ago, excluded patients that would be eligible for local treatment according to current guidelines [Citation7].
There is today compelling evidence that locally advanced and high-risk PC is a deadly disease regardless of age. A US study, based on SEER-Medicare data, demonstrated a 10-year cancer specific-mortality of 27% for patients older than 75 years with high-risk, localized PC [Citation15]. A Swedish nationwide register-based study found that even in patients older than 85 years, untreated, locally advanced PC with Gleason grade 8–10 was associated with a mortality rate of 42% [Citation16]. The authors suggest that “locally advanced tumors carry an entirely different prognosis and that considerably higher stakes may be justified in the choice of treatment.” A further study from Sweden showed clear underuse of radical therapy in elderly patients [Citation17]. In support of these findings, Sheng and colleagues found a positive impact of local treatment on cancer-specific mortality compared to conservative treatment in elderly patients (aged ≥ 75 years, locally advanced PC). However, the benefit was only seen in patients with cT3b/4 tumors, Gleason score 8–10, negative lymph nodes or PSA > 10 ng/ml [Citation18].
A recent publication of data from the National Cancer Database (NCDB) that captures approximately 70% of newly diagnosed cancers in the United States, documented that increasing age was significantly associated with a decreased likelihood of curative treatment in patients with intermediate and high-risk PC. Interestingly, despite the evidence of undertreatment, the study showed that more than half of patients older than 80 years had received some form of local treatment, mostly radiotherapy [Citation19]. This stands in contrast with treatment policies in Norway: In 2004 less than 5% of patients aged 75–79 years had received local treatment, with gradually increasing treatment numbers during the course of the following 12 years (∼50% local treatment in 2016). However, even in 2016 only slightly more than 10% of patients older than 80 years had received local therapy (radiotherapy, ≥70Gy, ≤12 months of diagnosis) [Citation14]. Similar findings are published from Sweden reflecting a Scandinavia-wide policy of stringent age criteria for offering local treatment a decade ago [Citation6]. This is surprising, as life expectancy for elderly men in Norway is high. For example, an 80-year-old Norwegian man had in 2018 an average life expectancy of 8.5 years (Source: Statistics Norway, www.ssb.no). Controlling localized high-risk or locally advanced PC over such prolonged periods of time without local treatment to the prostate may prove challenging.
The data presented in this study support this latter notion by highlighting another aspect of insufficient tumor control: Failure to offer local treatment may lead to considerable local complications during the further course of the disease. More than 80% of the elderly patients in our study experienced at least one complication that could be attributed to local tumor growth and a considerable number of patients had to live with long-term interventions such as catheterization, nephrostomies and colostomies.
As the median time from diagnosis to (prostate cancer) death in our population was only 7 years, the generally accepted policy at the time of offering local treatment only to patients with a life expectancy of more than 10 years seems inadequate. With hormone therapy, the median time to castration-resistance was 4 years. For many elderly patients, this is insufficient to avoid PC mortality and morbidity.
In terms of cancer morbidity other than local complications, almost half of the patients received blood transfusions. Only a minority received systemic second-or third-line therapy after the onset of castration-resistance (CRPC). This may reflect the functional status and health of patients who were unfit for treatment with docetaxel, as abiraterone and enzalutamide were not available at the time in Norway as first-line treatment for patients with CRPC. According to recent publications [Citation20,Citation21], it is reasonable to assume that considerably more elderly patients will be eligible for abiraterone and/or enzalutamide as first- and second-line treatment of CRPC. Thus, early local therapy may reduce not only tumor progression but also the occurrence of CRPC and consequently the need for a costly second- and third-line systemic therapy.
There is however an important caveat: Introducing routine local treatment of PC in elderly high-risk patients may create yet another area of overtreatment and potentially beneficial treatment effects must be weighed against side effects [Citation22]. Our study offers no indication of the scope of potential overtreatment. The role of routine local treatment in elderly high-risk and locally advanced NMPC patients with good functional status and health should thus be addressed by randomized trials and age as a sole exclusion criterion in this patient group ought to be abandoned.
With a retrospective design, there are obvious limitations to our study. However, the strength lies in the high quality of the data on comorbidity, functional status, local/systemic complications and other clinical information. Furthermore, the regional restriction and the small number of patients could be a weakness.
In summary, among NMPC patients who died of PC in Vestfold in 2009–2014 only a minority received local/curative therapy. For these patients, the lack of adequate local therapy had considerable consequences in terms of local tumor growth/morbidity and systemic complications. The study suggests that a more active approach in the use of local therapy in elderly patients with high-risk or locally advanced NMPC has the potential of reducing disease-related morbidity and mortality.
Ethical approval
The study was approved by the Regional Committee for Medical Research Ethics (REK, ID 2014/2203).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629.
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932–942.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. The Lancet. 2002;360(9327):103–108.
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308.
- Loffeler S, Halland A, Weedon-Fekjaer H, et al. High Norwegian prostate cancer mortality: evidence of over-reporting. Scand J Urol. 2018;52:122–128.
- Pettersson A, Robinson D, Garmo H, et al. Age at diagnosis and prostate cancer treatment and prognosis: a population-based cohort study. Ann Oncol. 2018;29(2):377–385.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU guidelines on prostate cancer. Arnhem the Netherlands: European Association of Urology; 2019.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- King JC, Zenati M, Steve J, et al. Deviations from expected treatment of pancreatic cancer in octogenarians: analysis of patient and surgeon factors. Ann Surg Oncol. 2016;23(13):4149–4155.
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. JCO. 2007;25(35):5570–5577.
- Rauh-Hain JA, Pepin KJ, Meyer LA, et al. Management for elderly women with advanced-stage, high-grade endometrial cancer. Obstet Gynecol. 2015;126(6):1198–1206.
- Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53(1):68–80.
- Årsrapport 2017. med resultater og forbedringstiltak fra Nasjonalt kvalitetsregister for prostatakreft. Oslo: Kreftregisteret (Norwegian Cancer Registry); 2018.
- Lu-Yao GL, Albertsen PC, Moore DF, et al. Fifteen-year outcomes following conservative management among men aged 65 years or older with localized prostate cancer. Eur Urol. 2015;68(5):805–811.
- Akre O, Garmo H, Adolfsson J, et al. Mortality among men with locally advanced prostate cancer managed with noncurative intent: a nationwide study in PCBaSe Sweden. Eur Urol. 2011;60(3):554–563.
- Bratt O, Folkvaljon Y, Hjalm Eriksson M, et al. Undertreatment of men in their seventies with high-risk nonmetastatic prostate cancer. Eur Urol. 2015;68(1):53–58.
- Sheng W, Kirschner-Hermanns R, Zhang H. Elderly patients aged ≥ 75 years with locally advanced prostate cancer may benefit from local treatment: a population-based propensity score-adjusted analysis. World J Urol. 2018;37:317–325.
- Yang DD, Mahal BA, Muralidhar V, et al. Receipt of definitive therapy in elderly patients with unfavorable-risk prostate cancer. Cancer. 2017;123(24):4832–4840.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148.
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377(2):132–142.
