Abstract
Background: This study investigated prostate cancer (PC)-specific survival and overall survival (OS) in a population-based castration-resistant PC (CRPC) cohort.
Methods: Data from Stockholm Prostate-Specific Antigen (PSA) and Biopsy Register patients with increasing PSA despite gonadotropin-releasing hormone treatment or surgical castration (n = 1,712) included PSA values and biopsies from 2003 to 2015 and were linked to the National Prostate Cancer Register and Prescribed Drug Register. Kaplan-Meier method estimated PC-specific survival and OS, stratified by metastasis at PC diagnosis, and Cox regression estimated hazard ratios (HRs) for Gleason score and T-stage at PC diagnosis and for age and calendar period at CRPC onset by metastasis status at diagnosis.
Results: Median OS after CRPC onset was 23.2 months (95% CI = 21.0–25.9) among patients without metastases (M0) at primary diagnosis, and 13.2 months (11.3–14.5) among patients with metastases (M1). Median PC-specific survival from CRPC onset was 30.3 (27.5–34.1) months and 13.3 (12.1–15.8) months for M0 and M1 patients, respectively. Biopsy Gleason score ≥ 8 was associated with higher all-cause mortality than ≤6 (HR = 2.07 [95% CI = 1.43–3.01]) and PC-specific mortality (2.07 [1.27–3.40]) after CRPC among patients with M0 disease. Patients developing CRPC from 2012 onward had lower all-cause mortality (HR = 0.71 [95% CI = 0.60–0.85] [M0]; 0.60 [0.47–0.77] [M1]) and PC-specific mortality (0.73 [0.57–0.94] [M0]; 0.62 [0.46–0.84] [M1]) compared with those prior to 2012.
Conclusions: M1 disease at PC diagnosis was associated with worse survival after CRPC onset versus M0. Higher Gleason score at diagnosis was associated with higher mortality after CRPC onset in M0 patients at diagnosis.
Introduction
Prostate cancer (PC) is the second most common cancer in men worldwide, accounting for 15% of cancers diagnosed in men [Citation1]. Androgen deprivation therapy (ADT) has historically been the standard of care for many patients across different stages of disease. Initial ADT achieves responses in nearly all patients; however, progression to castration-resistant disease or metastases within 5 years has been shown to be a marker for more aggressive disease [Citation2]. Men with high-risk non-metastatic castration-resistant PC (nmCRPC), characterized by rapidly rising prostate-specific antigen (PSA) despite castrate levels of testosterone, are at significant risk for development of metastases and PC-specific death [Citation3]. After treatment with docetaxel plus prednisone was shown to be effective for patients with metastatic CRPC (mCRPC) [Citation4], other therapies with positive results in clinical trials were introduced for treatment of mCRPC; these therapies included abiraterone acetate plus prednisone [Citation5–7], cabazitaxel [Citation8], enzalutamide [Citation9,Citation10], radium-223 [Citation11] and sipuleucel-T [Citation12]. Each of these agents has demonstrated improved overall survival in specific CRPC patient populations in randomized phase III trials. In 2018, based on clinical study results showing improved metastasis-free survival compared with ADT alone, two androgen receptor inhibitors, apalutamide and enzalutamide, were approved for use in patients with nmCRPC [Citation13,Citation14]. In another phase III trial in patients with nmCRPC, treatment with darolutamide significantly prolonged metastasis-free survival compared with placebo [Citation15].
Survival data for agents used in CRPC have come from randomized controlled studies; however, compared with mCRPC, limited data outside of the clinical trial setting are available for nmCRPC. Population-based PC outcomes, including predictors of time to metastases in CRPC [Citation16], treatment patterns in mCRPC [Citation17,Citation18] and clinical course in patients without metastases at initiating ADT [Citation19] have been described in multiple studies. However, there are limited data focusing on the impact of metastases at initial diagnosis on survival outcomes in CRPC outside of clinical trials [Citation20]. Understanding the real-world impact of metastases in CRPC has become especially important with the availability of recently approved antiandrogens for treating nmCRPC. In this retrospective, population-based study, the overall and prostate-specific survival among patients with CRPC in the real-world setting in Stockholm, Sweden were assessed.
Methods
Study population and patient databases
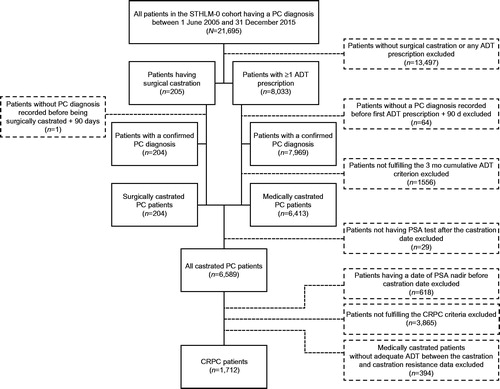
The Stockholm PSA and Biopsy Register (STHLM-0) is a population-based register that contains data on every PSA test and prostate biopsy taken in Stockholm County since 2003 [Citation21]. By linking these data to the National Cancer Register, the National Prostate Cancer Register, the Cause of Death Register and the National Prescribed Drug Register (in which only prescribed and expediated drugs are registered), a longitudinal cohort could be generated. These registers are maintained by the National Board of Health and Welfare in Sweden. The STHLM-0 cohort consists of ∼400,000 men and more than 1.8 million PSA tests. Based on the inclusion criteria described herein, all patients included in this study had a first PC diagnosis on or after 1 June 2005 and all patients entered the first CRPC phase on or after 1 January 2006 ().
Inclusion criteria
Men with a diagnosis of prostate cancer in the STHLM-0 cohort, who had rising PSA after meeting criteria for ADT or surgical castration (described below), were included in this analysis. The use of prescribed ADT was a prerequisite used to define medical castration.
Exclusion criteria
Patients with any of the following criteria were excluded: no surgical castration or no ADT prescription, no PC diagnosis recorded before the first ADT prescription plus 90 days, failure to fulfill the 3 months cumulative ADT criteria, no PSA record after the castration date, failure to fulfill the castration resistance criteria, a record of a nadir value before the castration date or no adequate ADT between the castration date and the castration resistance date (thereby excluding men receiving intermittent hormonal treatment).
Definitions used
Surgical castration
The castration criteria were fulfilled if a patient had a record of surgical castration in the Swedish National Prostate Cancer Register. The date associated with the procedure was used as the date of castration.
ADT criterion of 3 months’ cumulative use
For patients with at least one ADT record, treatment duration was calculated based on Anatomic Therapeutic Chemical code, dose and pack size. A patient was defined as being medically castrated if the patient had been cumulatively receiving ADT for at least 3 months within a total period of 6 months according to records in the Swedish National Prescribed Drug Register (Appendix).
Nadir PSA value and date
For patients who were identified as meeting the criteria for either ADT or surgical castration, PSA patterns were assessed in the STHLM-0 cohort. The nadir value was defined as the lowest observed PSA value after the start of ADT or surgical castration and the nadir date was the date for this PSA test.
Castration-resistant PC
We defined the date of entering castration resistance as the date of PSA testing when there was a doubling of nadir PSA value with the last value greater than 2 ng/ml or there was an absolute increase of 5 ng/ml or greater. Serum testosterone levels are not available in the database used in this study.
Compliance
ADT treatment was defined as adequate if the following two criteria were fulfilled: (1) coverage criteria – the total medication duration had to cover at least 40% of the time from the medical castration date to the CRPC date and during the rest of the follow-up; (2) compliance criteria – each medication duration had to cover at least 40% of the time from the current dispensation start date to next dispensation start date and the time from this estimated treatment end date to next dispensation start date had to be ≤ 220 days during the time of follow-up.
Cause of death
The cause of death for any patient who died during the follow-up period was identified using the Swedish Cause of Death Register. A death from PC was noted if the primary cause of death denoted by International Classification of Diseases (ICD) code was set for ‘prostate cancer’ denoted as ICD-10 = C619.
Statistical analyses
Baseline characteristics were provided and stratified by metastasis status at time of initial PC diagnosis (M1 = metastatic disease and M0 = non-metastatic disease [M0 included Mx at PC diagnosis]). The Kaplan–Meier method and 95% confidence intervals were used to estimate overall survival, PC-specific survival and median time to these events from onset of CRPC. Multivariable Cox regression models were used to assess all-cause- and PC-specific mortality for age at CRPC onset, T stage at PC diagnosis, Gleason score at diagnosis and calendar period at CRPC diagnosis (early: 2006–2011 vs late: 2012–2015). For these analyses, patients were stratified by their metastasis status at PC diagnosis.
Results
Patient characteristics
A total of 1,712 patients met the inclusion criteria (). The characteristics of these patients at the time of diagnosis or at the time of inclusion (specified in the table) are presented in . At the time of PC diagnosis, 1,123 (63%) patients had M0 disease and 467 (27%) patients had M1 disease. Of those patients with M0 disease at PC diagnosis, only 26 had undergone radical prostatectomy and 38 patients had been treated with any type of radiotherapy with curative intent. The mean and median PSA values at diagnosis were 399 and 85, respectively. When patients were stratified by metastasis status at PC diagnosis, the corresponding values were 238 and 69 [M0] and 791 and 214 [M1], respectively.
Table 1. Baseline patient and clinical characteristics of 1,712 patients in Sweden who had onset of CRPC during the years 2006–2015.
Overall survival and PC-specific survival for patients with CRPC
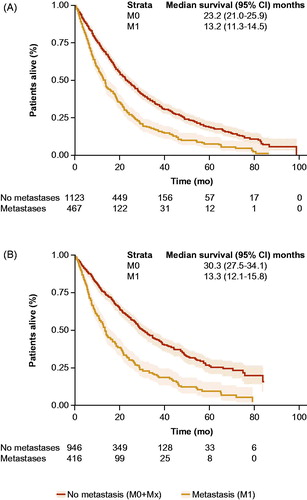
Median overall survival time after onset of CRPC for patients with or without metastases after the development of CRPC was 13.2 months (95% CI = 11.3–14.5) and 23.2 months (95% CI = 21.0–25.9), respectively (). The median PC-specific survival time after onset of CRPC for patients with or without metastases was 13.3 months (95% CI = 12.1–15.8) and 30.3 months (95% CI = 27.5–34.1), respectively ().
Figure 2. Kaplan–Meier method and 95% confidence intervals used to estimate overall survival (A) and PC-specific survival (B) and median time to these events from onset of CRPC. CRPC, castration-resistant prostate cancer.
Among patients with M0 disease at time of PC diagnosis, greater age (comparing patients aged >80 years with those <70 years [HR = 1.71; 95% CI = 1.37–2.12; p < 0.001]) and higher Gleason score (comparing Gleason ≥8 with Gleason ≤6 [HR = 2.07; 95% CI = 1.43–3.01; p < 0.001]) were associated with higher all-cause mortality in a multivariable model (). However, among men with M1 disease at PC diagnosis, a higher Gleason score at diagnosis was not associated with higher all-cause mortality after progressing to CRPC (). Among patients with M0 disease at the time of diagnosis, those who developed CRPC in the ‘later’ period, 2012–2015, had a lower all-cause mortality compared with those who developed CRPC in the ‘earlier’ period, 2006–2011 (HR = 0.71; 95% CI = 0.60–0.85; p < 0.001) (). Similarly, for those patients with M1 disease at time of PC diagnosis, development of CRPC in the later period (2012–2015) was associated with significantly lower mortality compared with development of CRPC in the earlier period (2006–2011) (HR = 0.60; 95% CI = 0.47–0.77; p < 0.001) ().
Figure 3. Hazard ratios from multivariable Cox regression model of all-cause mortality from onset of CRPC for age at PC diagnosis, T stage at PC diagnosis, Gleason score at PC diagnosis and calendar period of CRPC (early/late) by metastasis status at diagnosis (M0 [A] and M1 [B]). References for comparison were < 70 for age, T1 for stage, ≤ 6 for Gleason score, and 2006–2011 for year. CI, confidence interval; CRPC, castration-resistant prostate cancer; HR, hazard ratio; M0, non-metastatic disease; M1, metastatic disease.
![Figure 3. Hazard ratios from multivariable Cox regression model of all-cause mortality from onset of CRPC for age at PC diagnosis, T stage at PC diagnosis, Gleason score at PC diagnosis and calendar period of CRPC (early/late) by metastasis status at diagnosis (M0 [A] and M1 [B]). References for comparison were < 70 for age, T1 for stage, ≤ 6 for Gleason score, and 2006–2011 for year. CI, confidence interval; CRPC, castration-resistant prostate cancer; HR, hazard ratio; M0, non-metastatic disease; M1, metastatic disease.](/cms/asset/bb1b308d-7ed9-44d0-b6ab-9ce3ebeac4e3/isju_a_1739139_f0003_c.jpg)
PC-specific survival from CRPC was also assessed by metastasis status at PC diagnosis using multivariable models. Among patients with M0 disease, higher Gleason score was associated with increased mortality (comparing Gleason ≥ 8 with Gleason ≤ 6 [HR = 2.07; 95% CI = 1.27–3.40; p = 0.004]) (). For patients with M1 disease, high T stage at PC diagnosis was associated with higher PC-specific mortality (comparing stage 4 with stage 1 [HR = 1.72; 95% CI = 1.07–2.77; p = 0.025]) (). Development of CRPC in the later calendar period (comparing 2012–2015 with 2005–2011) was associated with lower PC-specific mortality for both M0 (HR = 0.73; 95% CI = 0.57–0.94; p = 0.013) and M1 (HR = 0.62; 95% CI = 0.46–0.84; p = 0.002) ().
Figure 4. Hazard ratios from multivariable Cox regression model of PC-specific mortality from onset of CRPC for age at PC diagnosis, T stage at PC diagnosis, Gleason score at PC diagnosis and calendar period of CRPC (early/late) by metastasis status at diagnosis (M0 [A] and M1 [B]). References for comparison were < 70 for age, T1 for stage, ≤ 6 for Gleason score and 2006–2011 for year. CI, confidence interval; CRPC, castration-resistant prostate cancer; M0, non-metastatic disease; M1, metastatic disease.
![Figure 4. Hazard ratios from multivariable Cox regression model of PC-specific mortality from onset of CRPC for age at PC diagnosis, T stage at PC diagnosis, Gleason score at PC diagnosis and calendar period of CRPC (early/late) by metastasis status at diagnosis (M0 [A] and M1 [B]). References for comparison were < 70 for age, T1 for stage, ≤ 6 for Gleason score and 2006–2011 for year. CI, confidence interval; CRPC, castration-resistant prostate cancer; M0, non-metastatic disease; M1, metastatic disease.](/cms/asset/b997346d-9eba-4fa4-a59d-58b5b6f94cdb/isju_a_1739139_f0004_c.jpg)
Discussion
In this population-based cohort of men with CRPC, those with metastases at the time of original PC diagnosis had inferior survival after the onset of CRPC than those with no metastases. We also found that a higher Gleason score at the time of diagnosis was associated with worse overall and cause-specific survival among CRPC patients with M0 disease at PC diagnosis but not among men with M1 disease upfront. Patients who were included in the study in later years also seemed to have lower overall and cause-specific mortality after the occurrence of CRPC, regardless of metastatic status at diagnosis, compared with patients who entered the study in early years. This result may, in part, be explained by truncated follow-up of the later cohorts, but could also be attributed to disease aggressiveness, introduction of newer agents for CRPC and the fine-tuning of methods used in clinical staging of patients.
It is somewhat surprising that men diagnosed with a Gleason 6 tumor are represented in this cohort, as these tumors are thought to not be able to metastasize or have a very low potential to differentiate into a more aggressive cancer. The most likely explanations for this were that most of these men were diagnosed in the years when sextant or octant prostate biopsies were the gold standard and there was a larger risk of being under-staged at the time of diagnosis, before the introduction of magnetic resonance imaging in the diagnosis of PC.
Biological interpretation
These data suggest that Gleason score at primary diagnosis is an indicator of overall and prostate-specific survival after CRPC among patients with non-metastatic at primary PC diagnosis. The level of differentiation of the primary tumor in patients with metastatic disease at PC diagnosis does not seem to affect the later course of the disease. This may be explained by several mechanisms. Patients having metastases at diagnosis clearly have a tumor with strong malignant potential regardless of the morphological signs of low differentiation and the Gleason score may, therefore, be less predictive. Possibly, under-grading of Gleason score may also be more prevalent among men with metastasized disease as it may be more clinically relevant to diagnose the prostate cancer by fewer biopsies rather than to do full systematic biopsies to cover the full prostate. Previous studies have reported an association between low Gleason score at diagnosis and a worse prognosis in the castration-resistant stage of disease [Citation16,Citation22], but we are unaware of studies that assessed the prognostic value of biopsy Gleason score by M stage in a cohort of men with CRPC.
Study limitations
Retrospective studies of population-based records have some inherent limitations due to available data within registers. In this study, no records on use of docetaxel or other chemotherapies were available for patients, as these are hospital-administered drugs and therefore not captured in the Prescription Drug Register in Sweden. In addition, some patients may have been enrolled in clinical studies for newer therapeutic agents and the analyses conducted did not control for patients participating in clinical trials. The present cohort is mainly from the era before early chemotherapy and novel antiandrogenic medications and the absolute survival times after entering castration resistance can only be generalized to patients of today not being fit for this type of medication.
According to the European Association of Urology, the definition of CRPC includes having a testosterone level of < 50 ng/dl or 1.7 nmol/l plus either three consecutive rises in PSA 1 week apart resulting in two 50% increases over nadir and a PSA >2 ng/ml or radiographic progression, defined as the appearance of new lesions in the bone or soft tissue. For this analysis, we did not have access to serum testosterone levels. In addition, only a fraction of the patients had three consecutive PSA samples taken within this short time span, thus reflecting the population-based setting in which the data were collected.
Study strengths
The strengths of this study comprise several facets, including the size of the cohort, the length of follow-up time and the completeness of data in the population-based registers [Citation23]. The STHLM-0 cohort enables longitudinal follow-up of detailed clinical variables with full coverage in the area of PC and the only loss in follow-up is death or emigration. Deaths without a record in the Cause of Death Register are uncommon. Furthermore, in Sweden, all patients with a cancer diagnosis are treated in publicly funded hospitals, which enables the possibility of equal treatment options being made available for all patients. This reduces the risk of missing information and selection bias. In addition, previous research has shown that the National Prostate Cancer Register of Sweden is of high quality [Citation23].
Conclusions
Our real-world observational study demonstrated that having M1 disease at PC diagnosis was associated with worse overall survival in the castration-resistant phase. In addition, higher Gleason score at diagnosis was associated with increased overall and PC-specific mortality in patients with M0 disease at PC diagnosis. Entering CRPC in the later calendar period was a predictor for superior overall and PC-specific survival from onset of CRPC. Overall, patients with M0 disease at the time of diagnosis of PC experienced longer survival from the onset of CRPC than those with M1 disease at the time of diagnosis. These findings confirm the negative impact of the development of M1 disease on patient outcomes in a real-world setting. Potentially owing to disease aggressiveness, the use of newer agents and/or the increased use of refined clinical staging methods, patients who developed CRPC in the later period (2012–2015) survived longer than those who developed CRPC in the earlier period (2006–2011). The recent availability of agents to treat patients with CRPC before the development of metastases has generated a renewed emphasis on earlier treatment. Trials with these agents have shown that they significantly delay disease progression in nmCRPC, which suggests that earlier treatment of patients with CRPC is highly beneficial [Citation13–15]. However, continued study is needed to more fully characterize CRPC, regardless of metastatic status, to better identify those patients who are most likely to benefit from earlier treatment. In addition, more study is needed to determine which treatments for CRPC provide the most benefit at earlier disease stages.
Acknowledgments
Writing assistance was provided by Keith Lantz, MS, CMPP, of Parexel and funding for editorial assistance was provided by Janssen Global Services, LLC.
Disclosure statement
Markus Aly, Tobias Nordström, Therese M.-L. Andersson, Chen Wang, Sandra Eloranta and Olof Akre report no conflicts of interest. Amy Leval and Johan Liwing are employees of Janssen-Cilag AB and may or may not hold stock in Johnson & Johnson. Frida Schain owns and is employed by Schain Research and was previously employed full time by Janssen and continues to work as a consultant for Janssen. Joe Lawson and Erik Sjöland are employees of Janssen Research & Development, LLC. Emese Vágó was previously an employee of Janssen.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386.
- Donovan MJ, Hamann S, Clayton M, et al. Systems pathology approach for the prediction of prostate cancer progression after radical prostatectomy. JCO. 2008;26(24):3923–3929.
- Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. JCO. 2005;23(13):2918–2925.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.
- Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–825.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–1418.
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–2474.
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–1246.
- Moreira DM, Howard LE, Sourbeer KN, et al. Predictors of time to metastasis in castration-resistant prostate cancer. Urology. 2016;96:171–176.
- Droz JP, Efstathiou E, Yildirim A, et al. First-line treatment in senior adults with metastatic castration-resistant prostate cancer: a prospective international registry. Urol Oncol. 2016;34(5):234.e21–9.
- Akaza H, Procopio G, Pripatnanont C, et al. Metastatic castration-resistant prostate cancer previously treated with docetaxel-based chemotherapy: treatment patterns from the PROXIMA prospective registry. JGO. 2018;4:1–12.
- Banefelt J, Liede A, Mesterton J, et al. Survival and clinical metastases among prostate cancer patients treated with androgen deprivation therapy in Sweden. Cancer Epidemiol. 2014;38(4):442–447.
- Westgeest HM, Uyl-de Groot CA, van Moorselaar RJA, et al. Differences in trial and real-world populations in the Dutch Castration-resistant Prostate Cancer Registry. Eur Urol Focus. 2018;4(5):694–701.
- Aly M, Clements M, Weibull CE, et al. Poor follow-up after elevated prostate-specific antigen tests: a population-based cohort study. Eur Urol Focus. 2019;5(5):842–848.
- Nakabayashi M, Hayes J, Taplin ME, et al. Clinical predictors of survival in men with castration-resistant prostate cancer: evidence that Gleason score 6 cancer can evolve to lethal disease. Cancer. 2013;119(16):2990–2998.
- Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. 2015;51(1):101–111.
Appendix
Algorithm to ensure criterion of 3 months’ use of ADT
The following algorithm was applied to calculate the date when the 3 months’ cumulative ADT criteria were fulfilled: for patients with the first ADT prescription duration longer than 3 months, the date when the 3 months’ treatment criteria were fulfilled was the start date of the first drug dispensation plus 90 days. For patients with the first ADT prescription shorter than 3 months and with a record of subsequent ADT prescription, the criteria were fulfilled if the combination of the first and second prescriptions corresponded to a treatment duration of cumulatively at least 3 months; the date when the 3 months’ treatment criteria was fulfilled was the start date of the second dispensation plus 90 days minus the first prescription duration. If the combination of the first and second prescription treatment durations was cumulatively shorter than 3 months, both the first and second prescriptions had to be at least 1 month and the actual date for fulfilling the 3 months’ treatment criteria was the start date of the third dispensation plus 30 days. Patients with an ADT dispensation record earlier than the end of the last dispensation were assigned the 3 months’ treatment date as the date for the first time of dispensation plus 90 days. For patients with an ADT dispensation record later than the end of the last dispensation, we allowed an additional 3 months’ period; consequently, we excluded patients who did not fulfill the 3 months’ treatment criteria in fewer than 180 days. The castration date was defined as the date when the 3 months’ treatment criteria was fulfilled.
Surgical castration definition
The hospital admission date related to the castration, as recorded in the Swedish National Patient Register, was used as an estimate of the actual surgery date (i.e. castration date).