Abstract
Background: Androgen deprivation therapy (ADT) is a non-curative but essential treatment of prostate cancer with severe side effects. Therefore, both over- and underuse should be avoided. We investigated adherence to guidelines for ADT following radical prostatectomy through Swedish population-based data.
Material and methods: We used the database Uppsala/Örebro PSA cohort (UPSAC) to study men with localised or locally advanced prostate cancer at diagnosis (clinical stage T1–T3, N0–NX, M0–MX, and prostate-specific antigen (PSA) <50 ng/ml) who underwent radical prostatectomy 1997–2012. 114 men were treated with ADT and selected as cases; 1140 men with no ADT at the index date were selected as controls within 4-year strata of year of radical prostatectomy. All men with a biochemical recurrence and a PSA doubling time <12 months and/or a Gleason score of 8–10 were considered to have an indication for ADT according to the European Association of Urology (EAU) guidelines.
Results: No indication for ADT was found in 37% of the cases. Among these, 88% had clinical stage T1–2 at diagnosis, 57% had a biopsy Gleason score 2–6, 98% had an expected remaining lifetime over 10 years, 12% received castration, and 88% received antiandrogen monotherapy. 2% of controls were found to have an indication for ADT, and 96% of these had an expected remaining lifetime over 10 years.
Conclusion: Our results indicate that overtreatment with ADT after radical prostatectomy is common, whereas undertreatment is unusual. Interventions to improve adherence to guidelines are needed to avoid unnecessary side-effects and long treatment durations with ADT.
Introduction
Androgen deprivation therapy (ADT) delays progression in advanced prostate cancer, but the benefit of ADT at asymptomatic biochemical recurrence after radical prostatectomy remains controversial [Citation1]. Early treatment with ADT has been found to delay progression to metastases among men with high-risk features [Citation2]. However, only a minority of the men with biochemical recurrence experience progression to metastases and death from prostate cancer [Citation3–8]. Overuse should be avoided since ADT has a range of adverse effects [Citation9–14]. European guidelines state that treatment with ADT at biochemical recurrence following radical prostatectomy should be reserved for men with the highest risk of progression, defined by a short prostate-specific antigen doubling time (PSADT), or a high Gleason score, and a long life expectancy [Citation15].
In a previous study we found that the projected durations of treatment with ADT in terms of castration after radical prostatectomy can be decades for men with low- and intermediate-risk tumours [Citation16]. However, we had not access to any variables to analyse the indications for treatment in that study. In the present study we link the start of ADT to prostate-specific antigen (PSA) data. Our aim was to assess the adherence to current guidelines with regards to tumour stage (T-stage), Gleason score, life expectancy, and ADT category to inform about possible over- and undertreatment. To our knowledge, this is the first study to assess the adherence to guidelines for treatment with ADT after radical prostatectomy.
Materials and methods
Population and data collection
The data was collected through the database Uppsala/Örebro PSA cohort (UPSAC), which comprises data on PSA testing from the clinical laboratories in five counties in the region of Uppsala/Örebro, a geographical area with approximately 1.4 million inhabitants. The database has a complete capture of PSA-tests measured during 2005–2014 for four of the counties and during 2006–2012 for one county. Through the unique personal identity number, the UPSAC is recordlinked to the National Prostate Cancer Register of Sweden (NPCR) and a number of other nationwide registers and demographic databases [Citation17]. The NPCR includes information on disease characteristics at diagnosis and primary treatment within 6 months from diagnosis. For this study we also used the links to the Prescribed Drug Register, the National Patient Register, the Cause of Death Register, the Register of the Total Population, and the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA). The verification of treatments followed the rules presented in PCBaSeTraject, a project based on NPCR and previously described in detail [Citation18].
Information on ADT treatment was collected through the Prescribed Drug Register, which started on July 1st, 2005 and links collected prescriptions to the personal identity number. We searched for the ATC-codes L02AA, L02AE, and L02BX02 to identify medical castration, while antiandrogen monotherapy was identified through the ATC-codes L02BB01-03. Treatment with an antiandrogen followed by castration within 3 months was regarded as flare protection and classified as castration. Surgical castration was identified through the ICD-codes KFC10 and KFC15 in the National Patient Register.
Design
We performed a population-based study of all men in the UPSAC database with localised and locally advanced prostate cancer at diagnosis (clinical stage T1–T3, N0–NX, M0–MX, and serum levels of PSA <50 ng/ml) who underwent radical prostatectomy as primary treatment 1997–2012. The men were only included if they had not received adjuvant or salvage radiotherapy before the date of inclusion.
We conducted a nested case/control study to assess both possible over- and undertreatment with ADT. The cases were defined as men who started treatment with ADT after radical prostatectomy during the period 1 January 2006 to 31 December 2014, and the date for start of ADT was defined as the inclusion date. Stratification into four strata was made based on year of radical prostatectomy, where each stratum covered a fixed period of 4 years. Within each stratum, 10 controls per case were selected based on the criteria of no treatment with ADT at the inclusion date.
PSADT was defined as the natural log of 2 divided by the slope of the linear regression line of the log of PSA over time [Citation3]. All measurements of PSA before and within 6 weeks after radical prostatectomy were excluded. The time window for PSA measurements for the cases was chosen as 1 year before the inclusion date. For the controls, the time window was chosen as 1 year before the last PSA measurement preceding the inclusion date. PSADT was calculated for all cases with two or more measurements of PSA ≥0.2 ng/ml [Citation19]. If only two PSA measurements were available and separated by less than four weeks, the last was excluded to reduce the risk of differential misclassification.
Family situation, educational level and comorbidities were measured at the date of inclusion. Educational level was classified according to years of schooling: low (≤9 years), middle (10–12 years) and high (≥13 years). Comorbidities were categorised according to the Charlson Comorbidity Index (CCI) by using the recordlink to the National Patient Register [Citation20]. The Regional Ethical Review Board at Uppsala University approved the study.
Definition of explanatory variables
The European Association of Urology (EAU) guidelines recommend early ADT after radical prostatectomy for men with a biochemical recurrence and a PSADT <6–12 months and/or a Gleason score 8–10, and a long life expectancy [Citation15]. In this study, we used one measurement of PSA ≥ 0.2 ng/ml measured six weeks or more after radical prostatectomy as a proxy for biochemical recurrence and considered this as a prerequisite for adherent treatment with ADT. We applied the PSADT criteria as PSADT <12 months. The Gleason score was accessible as prostatectomy specimen Gleason for 682 men in the study, since we lacked most of this information before 2007. The decision to treat with ADT was exclusively referred to the PSADT first, the prostatectomy specimen Gleason next, and the biopsy Gleason last. If any of these could explain the treatment decision, the treatment with ADT was considered adherent to guidelines.
Thus, we considered ADT for men with a PSADT ≥12 months, and/or a Gleason score ≤7, and/or without a biochemical recurrence as non-adherent to guidelines and described these men with regards to clinical T-stage, biopsy Gleason score, expected remaining lifetime, and ADT category. The controls with a biochemical recurrence and a PSADT <12 months and/or a Gleason score 8–10 were likewise considered as non-adherent and we described these men with regards to the same variables, except for ADT category.
We did a corresponding analysis based on the Swedish guidelines, which differs from the EAU guidelines by a cut-off at PSADT <6 months and a recommended observation for men with a PSADT >6 months until PSA rises to 5–10 ng/ml [Citation19]. In this analysis, the priority of the variables was set in the order: PSADT <6 months, prostatectomy specimen Gleason 8–10, biopsy Gleason 8–10, and PSA ≥5 ng/ml.
Expected remaining lifetime was considered as more than 10 years for the following combinations of age and comorbidity: <70 years and any CCI, 70–74 years and CCI ≤2, 75–79 years and CCI ≤1, and ≥80 years and CCI = 0.
Results
Characteristics of cases and controls
The characteristics at diagnosis and inclusion date of cases and controls are presented in . Totally 114 cases and 1140 controls were included. At diagnosis, the median PSA was 9 ng/ml (quartile (Q)1–Q3; 6–15) for the cases while it was 7 ng/ml (Q1–Q3; 5–10) for the controls. The cases had higher T-stage and Gleason score at diagnosis than the controls. At inclusion date, the median PSA was 3 ng/ml (Q1–Q3; 1–9) for the cases and 0.1 ng/ml (Q1–Q3; 0.03–0.1) for the controls. The cases had a higher age at the inclusion date than the controls. Educational level, marital status, and comorbidity index were similarly distributed. Among the cases 27% were treated with castration and 73% were treated with antiandrogen monotherapy.
Table 1. Characteristics of the study population.
Adherence to the EAU guidelines
In the analysis of adherence to the EAU guidelines, we defined 59% of the cases as adherent to the guidelines, as they had a biochemical recurrence and a PSADT <12 and/or a Gleason score of 8–10. We defined 37% of the cases as non-adherent, as the treatment decision could not be explained by neither PSADT nor Gleason score, or no biochemical recurrence was found. The proportion where no PSA was available for analysis was 4% (). Among the non-adherent cases, 88% had T-stage 1–2 at diagnosis, 57% had a biopsy Gleason score 2–6, and 98% had an expected remaining lifetime over 10 years. In this group 12% received castration and 88% received antiandrogen monotherapy ().
Figure 1. Adherence to the European Association of Urology (EAU) guidelines and the Swedish guidelines for androgen deprivation therapy following radical prostatectomy. Proportion of men who had a PSA ≥ 0.2 ng/ml (PSA relapse) and at least one of the following at the start of androgen deprivation therapy (ADT): a PSADT <6 months, and/or a PSADT 6–12 months, and/or a radical prostatectomy specimen Gleason score 8–10, and/or a biopsy Gleason score 8–10, and/or a PSA ≥5 ng/ml. If the criteria above were fulfilled, the proportion was labelled as adherent for the cases and non-adherent for the controls. Proportion of men with no PSA-tests. Proportion of men who had a PSA relapse and none of the above criteria at the start of ADT. Proportion of men with no PSA relapse at the start of ADT. PSA: prostate-specific antigen; PSADT: PSA doubling time; RP-GS: radical prostatectomy specimen Gleason score; GS: biopsy Gleason score.
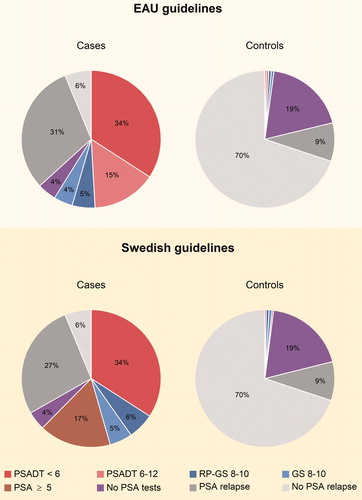
Table 2. Characteristics of the cases.
Among the controls 2% fulfilled the criteria of a biochemical recurrence and a PSADT <12 months and/or a Gleason score of 8–10 without receiving ADT, while 79% did not fulfil these criteria. The proportion where no PSA was available for analysis was 19% (). Among the controls with an indication for ADT, none had T-stage 3 at diagnosis, 33% had a biopsy Gleason score 8–10, and 96% had an expected remaining lifetime over 10 years ().
Table 3. Characteristics of the controls.
Adherence to the Swedish guidelines
In the analysis of adherence to the Swedish guidelines, 62% of the cases were labelled as adherent as they had a biochemical recurrence and a PSADT <6 months, and/or a Gleason score of 8–10, and/or a PSA ≥5 ng/ml. For 33% of the cases no indication for ADT was found in terms of PSADT, Gleason score, or PSA level, or no biochemical recurrence was found (). Among the non-adherent cases 89% had T-stage 1–2 at diagnosis, 50% had a biopsy Gleason score 2–6, 92% had an expected remaining lifetime over 10 years, 11% received castration, and 89% received antiandrogen monotherapy ().
Among the controls, 2% were found to have an indication for ADT as they had a biochemical recurrence and a PSADT <6 months and/or a Gleason score of 8–10, and/or a PSA ≥5 ng/ml, while 79% did not fulfil these criteria (). Among these men, 4% had T-stage 3 at diagnosis, 35% had a biopsy Gleason score 8–10, and 87% had an expected remaining lifetime over 10 years ().
Discussion
This population-based observational study indicates that overtreatment with ADT is common, since approximately one man out of three who receive ADT after radical prostatectomy have a PSADT ≥12 months and a Gleason score ≤7, or no biochemical recurrence. Our data also indicate that undertreatment is uncommon, since only two men out of hundred have a biochemical recurrence and a PSADT <12 months or a Gleason score 8–10 without receiving ADT. Antiandrogen monotherapy is the dominating form of ADT following radical prostatectomy in Sweden.
Approximately one man out of three received ADT in non-adherence to guidelines as the indications for treatment could be defined by the data in our study. Almost all of these men had an expected remaining lifetime over 10 years, which fits the guideline criteria of a long life expectancy. However, the majority also had a biopsy Gleason score 2–6 and T-stage 1–2 at diagnosis. The natural history after biochemical recurrence is recognised as highly variable, and the median time between biochemical recurrence and systemic progression range from 1 year to over 15 years, depending on risk features [Citation5,Citation7]. Therefore, many men with low-risk cancers never proceed to experience metastatic disease, but die of other causes. In these cases, treatment with ADT is likely to cause more harm than benefit and is non-adherent to guidelines [Citation15,Citation19].
One possible barrier to adherence in our study is the conflicting evidence of treatment with ADT after radical prostatectomy, which adds complexity to the guidelines and may cause uncertainty among clinicians [Citation21,Citation22]. The importance of the physician is highlighted in an observational study by Shahinian et al, which found that initiation of ADT depends more on the urologist than on tumour and patient characteristics [Citation22]. Therefore, interventions targeted towards urologists may be effective to improve the adherence to guidelines for ADT [Citation22,Citation23]. However, prostate cancer anxiety and low health literacy among patients have also been identified as predictors for early use of ADT after curative treatment [Citation24,Citation25]. Interventions working at multiple levels to improve adherence have been evaluated in several systematic meta-reviews, concluding that more knowledge is needed on how to efficiently tailor these interventions [Citation21,Citation26,Citation27].
Among the controls two men out of hundred fulfilled the indications for ADT according to the guidelines, and one third of them had a biopsy Gleason score of 8–10. Nearly all of these men had an expected remaining lifetime over 10 years, why this finding indicates undertreatment. This proportion is so small that it is unlikely that it represents a systematic misapplication of guidelines. An explanation for the possible undertreatment in our study can be intentional non-adherence. The EAU has a disclaimer for the guidelines, stressing that a guideline is not meant to replace physician judgment in treating particular patients on a case-by-case basis [Citation15]. Common reasons for intentional non-adherence to guidelines has been identified as presence of contra-indications or comorbidities, and patient preference [Citation21,Citation28]. Given that ADT has a range of side-effects, patient reluctance towards the treatment could partly explain non-adherence.
Antiandrogen monotherapy was the most used form of ADT in our study. In the Swedish guidelines, bicalutamide is recommended as the first-hand choice of ADT after radical prostatectomy for non-metastatic disease [Citation19]. The EAU guidelines state that antiandrogen monotherapy has not been shown to be inferior to castration for men with non-metastatic disease, but gives no explicit recommendation [Citation15,Citation29]. Castration is recognised to have more severe side effects than anti-androgens, such as hot flashes, sexual dysfunction, decreased bone density, decreased insulin sensitivity, increased risk of cardiovascular and thromboembolic disease, and adverse psychological effects [Citation9–14]. The most common adverse effects during treatment with bicalutamide are recognised as breast pain and gynecomastia, which can be avoided through prophylactic breast irradiation [Citation30,Citation31]. Thus, the consequences of overuse of ADT are probably more severe in countries where castration is more often used than antiandrogen monotherapy for non-metastatic disease.
The foremost strength of our study is the high capture rate of the registries. The PSA database has a complete capture rate for the studied time periods. NPCR captures 98% of all men with prostate cancer in the Swedish Cancer Registry, to which registration is mandated by law [Citation17]. One limitation is that the histo-pathological Gleason score from surgical specimens partly had to be replaced by biopsy Gleason score in our study. We had information on prostatectomy specimen Gleason score for 60 of the 114 cases. Among these, 52 men had a biopsy Gleason ≤7 and 13 of them (25%) were upgraded to specimen Gleason 8–10. Accordingly, eight men had a biopsy Gleason 8–10 and two of them (25%) were downgraded to specimen Gleason ≤7. This implicates that the lack of information on prostatectomy specimen Gleason score had a modest impact on the study results. Another limitation is that we have not evaluated the adherence to the guidelines for salvage radiotherapy. It should therefore be recognised that both cases and controls in our study could have been undertreated with regards to salvage radiotherapy, which has curative potential for men with a local recurrence after radical prostatectomy [Citation15,Citation19].
Conclusions
Our results indicate that overtreatment with ADT after radical prostatectomy is common, whereas undertreatment is unusual. Overtreatment with ADT implicates unwarranted exposure to side effects, while undertreatment may lead to shorter time to progression and death. Interventions to improve adherence to guidelines for ADT after radical prostatectomy are needed to avoid unnecessary side-effects and long treatment durations with ADT.
Acknowledgements
This study was made possible by the work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Ingela Frank Lissbrant, Camilla Thellenberg, Johan Styrke, Hampus Nugin, Lennart Åström, Stefan Carlsson, Marie Hjälm-Eriksson, David Robinson, Mats Andén, Ola Bratt, Maria Nyberg, Olof Ståhl, Tomas Jiborn, Hans Joelsson, Gert Malmberg, Olof Akre, Per Fransson, Johan Stranne, Jonas Hugosson, Eva Johansson, Magnus Törnblom, Fredrik Jäderling, Fredrik Sandin, Karin Hellström. The work of the clinical laboratories in the region of Uppsala/Örebro was indispensable for the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Van den Bergh RCN, van Casteren NJ, van den Broeck T, et al. Role of hormonal treatment in prostate cancer patients with nonmetastatic disease recurrence after local curative treatment: a systematic review. Eur Urol. 2016;69:802–820.
- Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–1147.
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer–specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439.
- Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109:32–39.
- Brockman JA, Alanee S, Vickers AJ, et al. Nomogram predicting prostate cancer–specific mortality for men with biochemical recurrence after radical prostatectomy. Eur Urol. 2015;67:1160–1167.
- Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59:893–899.
- D’Amico AV, Moul J, Carroll PR, et al. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172:42–47.
- Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–836.
- Thorstenson A, Bratt O, Akre O, et al. Incidence of fractures causing hospitalisation in prostate cancer patients: results from the population-based PCBaSe Sweden. Eur J Cancer. 2012;48:1672–1681.
- Crawley D, Garmo H, Rudman S, et al. Association between duration and type of androgen deprivation therapy and risk of diabetes in men with prostate cancer. Int J Cancer. 2016;139:2698–2704.
- O'Farrell S, Garmo H, Holmberg L, et al. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243–1251.
- O'Farrell S, Sandström K, Garmo H, et al. Risk of thromboembolic disease in men with prostate cancer undergoing androgen deprivation therapy. BJU Int. 2016;118:391–398.
- Donovan KA, Walker LM, Wassersug RJ, et al. Psychological effects of androgen-deprivation therapy on men with prostate cancer and their partners. Cancer. 2015;121:4286–4299.
- European Association of Urology. Prostate Cancer. [accessed 2020 Jan 5]. Available from: https://uroweb.org/guideline/prostate-cancer/#6_3
- Lycken M, Garmo H, Adolfsson J, et al. Patterns of androgen deprivation therapies among men diagnosed with localised prostate cancer: a population-based study. Eur J Cancer. 2014;50:1789–1798.
- The National Prostate Cancer Register of Sweden. English. [accessed 2020 Jan 5]. Available from: http://npcr.se/in-english/
- Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort profile update: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base—a refined prostate cancer trajectory. Int J Epidemiol. 2016;45:73–82.
- Regionala cancercentrum i samverkan. Vårdprogram. [accessed 2020 Jan 5]. Available from: https://www.cancercentrum.se/samverkan/cancerdiagnoser/prostata/vardprogram/
- Kastner C, Armitage J, Kimble A, et al. The Charlson comorbidity score: a superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis. 2006;9:270–274.
- Francke AL, Smit MC, de Veer AJ, et al. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38.
- Shahinian VB, Kuo YF, Freeman JL, et al. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98:839–845.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465.
- Dale W, Hemmerich J, Bylow K, et al. Patient anxiety about prostate cancer independently predicts early initiation of androgen deprivation therapy for biochemical cancer recurrence in older men: a prospective cohort study. J Clin Oncol. 2009;27:1557–1563.
- Mahal BA, Chen MH, Bennett CL, et al. High PSA anxiety and low health literacy skills: drivers of early use of salvage ADT among men with biochemically recurrent prostate cancer after radiotherapy? Ann Oncol. 2015;26:1390–1395.
- Flodgren G, Hall AM, Goulding L, et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev. 2016:CD010669.
- Baker R, Camosso-Stefinovic J, Gillies C, et al. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010:CD005470.
- Arts DL, Voncken AG, Medlock S, et al. Reasons for intentional guideline non-adherence: a systematic review. Int J Med Inf. 2016;89:55–62.
- Kunath F, Grobe HR, Rücker G, et al. Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev. 2014:CD009266.
- Iversen P, Tyrrell CJ, Kaisary AV, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol. 2000;164:1579–1582.
- Mcleod DG, Iversen P, See WA, et al.; the Casodex Early Prostate Cancer Trialists’ GroupP. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97:247–254.
