Abstract
Introduction: Novel drugs have been shown to prolong life in men with metastatic prostate cancer (PCa) and castration resistant Pca (CRPC). The aim of Patient-overview Prostate cancer (PPC) is to register and report these treatments and their effect.
Material and methods: In PPC, a new part of the National Prostate Cancer Register of Sweden data on start and stop of treatments, imaging, prostate specific antigen, clinical assessment of progression and patient reported outcome measures (PROM) are registered from initiation of hormonal treatment. Data are displayed in a graph to inform clinical decisions for individual patients. For research, data in PPC are linked to PCBaSe with information from NPCR and a number of health care registers.
Results: In December 2019, 7 882 men had been registered in PPC out of whom 3 912 had reached the CRPC state. Median time to start of androgen receptor targeted drugs (ART) from start of ADT was 4 years (interquartile range IQR 6) for men with primary ADT, and 9 years (IQR 6) and for men with secondary ADT. Out of all men in PCBaSe with a prescription for ART in 2016-2017, PPC captured 1 480/4 055 (36%). There were small differences between men registered/not registered in PPC for cancer characteristics, primary treatment, comorbidity, and time on ADT before start of ART.
Conclusion: In PPC, use and effects of novel therapies for advanced Pca are assessed in a real-life setting. PPC data are used as a decision aid, for quality assurance, and in research.
Introduction
Several drugs have recently been shown to prolong life and increase quality of life in men with metastatic prostate cancer (Pca) and castration resistant prostate cancer (CRPC) in randomized clinical trials (RCT) [Citation1]. These treatments include taxane-based chemotherapy, androgen receptor targeting (ART) drugs; abiraterone and enzalutamide, an alpha-emitting radionucleid; radium -223 and further drugs will be introduced in the near future [Citation2]. Since the study populations in RCT´s differ from afflicted men in the general population, there is a need for data from clinical practice, i.e. in a real-life setting, to assess if results obtained in RCTs are applicable to men with metastatic Pca and CRPC in the general population [Citation3]. There is also a need to assess if treatments are used according to guidelines in order to avoid over and underuse of new, expensive drugs and there is a need for a user-friendly display of data for each patient with advanced Pca.
The aim of this study was to delineate the set-up and to report preliminary results of The Patient-overview Prostate Cancer (PPC), a new part of the National Prostate Cancer Register (NPCR) of Sweden that was created in order to longitudinally register data from routine health care of men with advanced Pca.
Materials and methods
Primary registration in National Prostate Cancer Register of Sweden
The National Prostate Cancer Register (NPCR) of Sweden records a comprehensive set of data on cancer characteristics, diagnostic work-up, primary treatment, and primary treatments. In addition, secondary radical procedures i.e. prostatectomy and radiotherapy delivered after an initial period of active surveillance or radiotherapy after primary prostatectomy are also registered [Citation4–6]. Since 1998, NPCR captures 98% of all incident cases of Pca compared to the National Cancer Registry to which reporting is mandated by law. However, except registration of second line radical radiotherapy and prostatectomy, no further registration after the initial work up and primary treatment is made in NPCR. Since 2014, data in NPCR are uploaded in a dash board panel available to each care provider at a secured server, and as of recently also to the general public in an interactive reporting system [Citation7,Citation8].
Prostate Cancer data Base Sweden
In 2009, Prostate Cancer data Base Sweden (PCBaSe) was created by linking NPCR to a number of national health care registries and demographic databases, among these the Patient Registry with data on discharge diagnoses as of 1987, The Prescribed Drug Registry with data since July 2005, and The Cause of Death Registry [Citation5]. Subsequently, PCBaSe has been enriched by inclusion of additional sources and further curated [Citation6]. PCBaSe 4.0, the most recent update performed in 2016, includes more than 180 000 prostate cancer cases and almost 1 million prostate cancer-free control men frequency-matched on birth year and county of residency. Follow-up can be made by use of data in the Patient Registry on treatments and hospital admissions related to disease and disease progression and data on filled prescriptions in Prescribed Drug Registry, e.g. use of androgen deprivation therapy (ADT).
Patient overview Prostate Cancer (PPC)
PPC was created in order to overcome the lack of follow-up data in NPCR and PCBaSe on PSA progression, drug treatment with chemotherapy and radionucleids provided in-hospital (not available in the Prescribed Drug Registry), imaging procedures, and data on patient reported outcome measures (PROM) for men with advanced Pca.
Men who start ADT for Pca are eligible to be registered in PPC and for men who already had reached the CRPC state at the time of inclusion in PPC, selected data from the date of ADT and onwards are retrieved from medical charts and entered to PPC.
In PPC information is captured from the date of initiation of ADT to the date of death. Data that are registered include date for CRPC, dates for start and stop of Pca treatments, results from imaging investigations, laboratory tests e.g. serum levels of prostate specific antigen (PSA) and alkaline phosphatase, symptomatic skeletal-related events, and clinical assessment of clinical cancer status by use of The Eastern Cooperative Oncology Group/World Health Organization Performance Status [Citation9,Citation10]. These data are entered to PPC by staff and treating physician at each outpatient visit. Since beginning of 2018, a modified version of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C15-PAL questionnaire on Patient Reported Outcome Measures (PROM) is used before each visit, with the addition of a question concerning urinary symptoms [Citation11]. Since October 2018, EQ5D questionnaires, version EQ-5D-5L, are also collected for analysis of health economics [Citation12,Citation13]. PPC is held at the Information network on cancer care (INCA) platform, which also holds data for all clinical cancer registers in Sweden [Citation7].
Data from PPC reported here were included in update of PCBaSe, performed in February 2018. By use of these linkages, data on cancer characteristics at diagnosis, primary treatment, filled prescriptions, comorbidity assessed by use of the Charlson Comorbidity Index (CCI) based on discharge diagnoses from hospital admissions, level of education, sick leave, occupation, marital status, and cause of death are available for men in PPC [Citation6].
Results
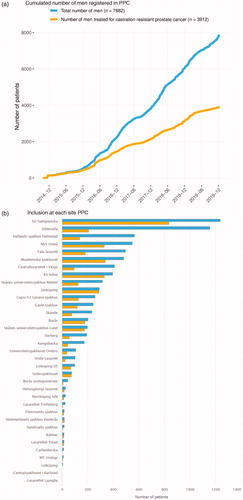
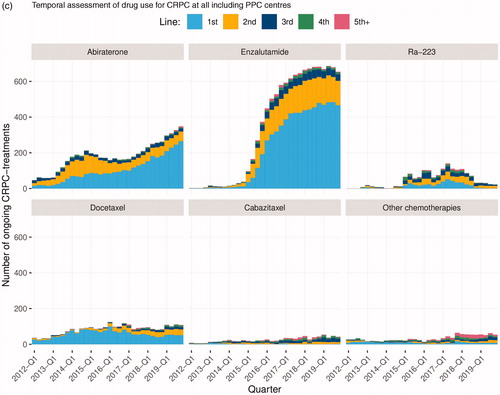
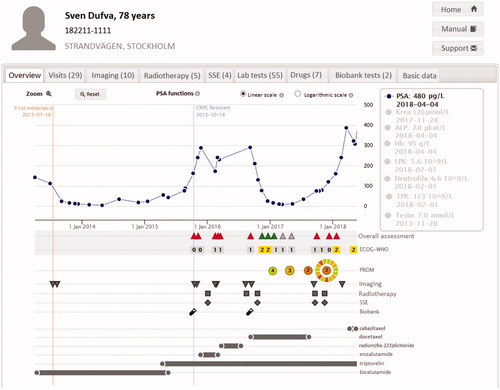
In December 2019, 7 882 men had been registered in PPC out of whom 3 912 had reached the CRPC state (). Data registered in PPC are displayed in a graph for each individual patient showing a time line with treatments and treatment effects including symptoms and quality of life (). This graph is used to inform decisions on treatment and follow-up by the physician and the patient at each consultation. A hard copy of the dashboard panel can readily be printed for the patient. Second, at each participating department, staff can access the PPC online interactive reporting system on the INCA platform. For example, data that can be readily analyzed by use of this reporting system include inclusion rate over time (), number of included patients per site (), use of drugs according to line of sequence (), other specific number of treated men over time as well as various specific request by individual sites.
Figure 1. (a) Cumulated number of men registered in the Patient-overview Prostate Cancer (PPC). (b) Inclusion at each site Patient-overview Prostate Cancer (PPC). (c) Temporal assessment of drug use for castration resistant prostate cancer (CRPC) at all including PPC centres in Sweden.
Figure 2. Graph in PPC with longitudinal overview of treatment effects including clinical assessment and Patient Reported Outcome Measures (PROM).
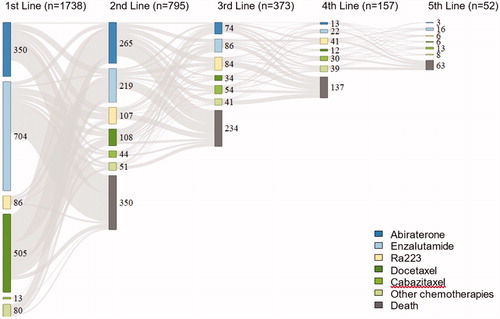
Data in PPC can thus be used to assess drug use at a hospital, in a county, in a region, or in the entire nation over time. As an example, shows the registration over time in PPC of use of androgen receptor targeted (ART) drugs, Ra-223, and chemotherapies in all participating departments as first to fifth line of treatment. The use of enzalutamide and abiraterone increased substantially between 2012 and 2018, whereas the use of Ra-223 decreased and the use of docetaxel decreased somewhat. There was only a small fraction of men who received cabazitaxel or other chemotherapies. The sequence of drugs, i.e. in which order the drugs were used is illustrated in . The most common first line therapy in men with CRPC in PPC was enzalutamide, followed by docetaxel and abiraterone, and the most common second line treatment was abiraterone. Out of the 1 738 men who received a first line treatment, 795 (46%) received a second line treatment, 373 (21%) received a third line treatment 157 (9%) received a fourth line treatment and 52 (3%) received a fifth line treatment.
shows data on men in PPC who were also included in PCBaSe 4.0. Data in PPC enriched by data in PCBaSe 4.0 enabled us to assess baseline characteristics at date of diagnosis and at date of start of androgen receptor targeted (ART) drugs. Overall, 1 586/3 417 (46%) of these men had regionally or distant metastatic Pca at date of diagnosis. Men who received primary ADT, indicating an advanced disease stage already at the date of diagnosis, were older at date of diagnosis than men who received other primary treatments, median age 71 years (interquartile range IQR 11) years vs 66 years (IQR 10). Primary ADT was used for a shorter period of time before start of ART than in men with secondary ADT, 4 years (IQR 5) years vs 9 years (IQR 6) . Men with primary ADT had higher median PSA at start of ART than men with secondary ADT, 84 ng/ml (IQR 185) vs 55 ng/ml (IQR 150).
Table 1. Characteristics of men in Patient -overview Prostate Cancer (PPC) at date of prostate cancer diagnosis, date of start of secondary androgen deprivation therapy by use of data in Prostate Cancer data Base (PCBaSe) v 4.1 and at date of start of androgen receptor targeted drug by use of data in PPC according to primary treatment.
The capture rate of men on ART in PPC could be assessed since filled prescriptions for these drugs are recorded in the Prescribed Drug Registry for all men in Sweden and hence for all men in NPCR. Out of all men in NPCR who filled a prescription for an ART in 2016-2017, PPC captured 1 480/4 055 (36%) of these men (). There were small differences between men registered in PPC and men registered in NPCR but not registered in PPC regarding cancer characteristics at diagnosis, primary treatment, comorbidity, and time on ADT before start of ART. For example, median age at date of diagnosis was the same, 68 years (IQR 11), distribution of risk categories and socioeconomic status at date of diagnosis was similar, median time from start of ADT to start of ART was the same, 4 years (IQR 5), and CCI was 0 at start of ART for 58% of men in PPC and 60% for men not in PPC.
Table 2. Characteristics of men in Prostate Cancer database Sweden (PCBaSe) 4.1 treated with Androgen Receptor Targeted drugs in 2016–2017 according to filled prescriptions in the Prescribed Drug Registry and stratified according to registration or not in Patient-overview Prostate Cancer (PPC).
To compare outcome in PPC with outcome in a RCT, we compared the decrease in serum levels of alkaline phosphatase (ALP) as a response to Ra -223 in PPC with the decrease in the ALSYMPCA trial [Citation14]. In that trial, 59% of men treated with Ra-223 lowered their serum ALP with more than 30% at 12 weeks of follow-up, whereas in PPC, 54% of men treated with Ra- 223 had a decrease in ALP of more than 30% at a median of nine weeks of follow-up (Supplemental Figure 1).
Discussion
Data in the Patient-overview Prostate Cancer (PPC) a new part of the National Prostate Cancer Register (NPCR) of Sweden, on use and effects of cancer treatments can be used for multiple purposes. Data are displayed in a graph that is used to inform decisions for individual patients at each consultation and data are also used for surveillance of drug use and effects and quality assurance at each department, for benchmarking between departments, and in research.
Limitations of PPC include that follow-up is still quite short and that PPC currently only captures a fraction of all men with advanced Pca in Sweden, currently estimated to be around one third of men with treated with an androgen receptor targeted (ART) drug, i.e. abiraterone or enzalutamide.
Strengths of PPC include that the denominator, i.e. all men with Pca in Sweden, is known by use of data in the primary registration in NPCR, since NPCR captures 98% of all men with prostate cancer in the Swedish Cancer Registry, to which reporting is mandated by law. In PCBaSe, NPCR including PPC has been linked to a number of other population-based nationwide health care registers and demographic databases so by comparing characteristics of men in PPC with all men in PCBaSe, the representativity of PPC can be assessed for a number of aspects including cancer characteristics at date of diagnosis, comorbidity, and socioeconomic status.
We assessed the proportion of men in NPCR with a filled a prescription for abiraterone or enzalutamide in the Prescribed Drug Registry who had also been registered in PPC and found that one third out of all these men in NPCR were also registered in PPC. Characteristics at baseline and at date of start of ART for men in PPC and for men in PCBaSe but not in PPC were very similar. Therefore, we argue that men on ART in PPC are representative of all men on ART in Sweden. Furthermore, we argue that PPC can be used to assess the representativity of RCT´s in a general population. As an example, we compared the short -term decrease in serum levels in alkaline phosphatase after Ra-223 treatment in the ALSYMPCA trial with the results in PPC and found that the results were similar in PPC compared to results in the ALSYMPCA trial [Citation14].
Another area in which data in PPC can be used is post authorisation safety studies (PASS). By use of data in PPC on drug exposure also including drugs delivered in-hospital that are not registered in The Prescribed Drug Registry, combined with outcome data e.g. hospital admissions due to rare adverse events in the Patient Registry available in PCBaSe, a cost-efficient PASS can readily be performed.
Compared to a large majority of conventional electronic medical charts, the graph in PPC provides a superior overview of a patient´s disease trajectory that often spans over a very long time period. Another asset in PPC is that PROM data are collected before each visit and these data are collated and displayed in a graph immediately available for the consultation. Currently, similar patient overviews are constructed on a generic platform at INCA for a number of other cancer sites other than Pca including renal cell carcinoma, lung cancer, breast cancer, CNS tumors, ovarian cancer, and myeloma in a project managed by the Federation of Regional Cancer Centers. In the future, patient overviews will hopefully be a part of the electronic medical record system, alternatively that patient overviews are seamlessly integrated with the medical record allowing users to easily access the patient overview from within the medical record of an individual patient’s record.
Another area in which patient overviews can become a cost-efficient tool is register -based RCT´s [Citation15]. As a first step PPC can be used to quickly identify men that could be potentially eligble by applying the relevant inclusion criteria and PPC may with some adjustments be used as a clinical report form.
In conclusion, there is a need for a structured registration of data in cancer care to be used as an aid in patient-centered clinical work, as metrics for quality assurance, and quality improvement and for research. To serve these purposes, a new part of NPCR; the Patient-overview Prostate Cancer (PPC) was constructed.
Fig_SUPPL_1_tif__1_.tif
Download TIFF Image (106.2 KB)Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chair), Ingela Franck Lissbrant (deputy chair), Johan Styrke, Camilla Thellenberg Karlsson, Lennart Åström, Eva Johansson, Stefan Carlsson, Marie Hjälm-Eriksson, Magnus Törnblom, Olof Akre, David Robinson, Mats Andén, Ola Bratt, Johan Stranne, Jonas Hugosson, Maria Nyberg, Per Fransson, Fredrik Sandin, Karin Hellström, Calle Waller and Gert Malmberg. We thank Anna Cedvall Gustavsson, David Örtquist, and Jonas Celander-Guss for IT support, and Maria Nyberg and Nina Hageman for administrative support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645–657.
- Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: Management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75(1):88–99.
- Saturni S, Bellini F, Braido F, et al. Randomized controlled trials and real life studies. Approaches and methodologies: a clinical point of view. Pulm Pharmacol Ther. 2014; 27(2):129–138.
- National Prostate Cancer Register. [cited 2020 Jan 9]. Available from: https://www.npcr.se.
- Hagel E, Garmo H, Bill-Axelson A, et al. PCBaSe Sweden: a register-based resource for prostate cancer research. Scand J Urol Nephrol. 2009;43(5):342–349.
- Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort profile update: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base–a refined prostate cancer trajectory. Int. J. Epidemiol. 2016;45(1):73–82.
- Stattin P, Sandin F, Sandbäck T, et al. Dashboard report on performance on select quality indicators to cancer care providers. Scand J Urol. 2016;50(1):21–28.
- Stattin P, Sandin F, Loeb S, et al. Public online reporting from a nationwide population-based clinical prostate cancer register. BJU Int. 2018;122(1):8–10.
- Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- The Eastern Cooperative Oncology Group/World Health Organization Performance Status. [cited 2020 Jan 9]. Available from: https://www.mdcalc.com/
- Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006; 42(1):55–64.
- EQ5D. [cited 2020 Jan 9]. Available from: https://euroqol.org.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223.
- James S, Rao SV, Granger CB. Registry-based randomized clinical trials–a new clinical trial paradigm. Nat Rev Cardiol. 2015;12(5):312–316.