Abstract
Objective: The primary aim of this study was to evaluate the scientific evidence supporting the use of thermal dose as a secondary (or an alternative) endpoint when using the CoreTherm Concept.
Material and methods: Baseline and treatment data from 283 consecutive treatments were evaluated. All patients had lower urinary tract symptoms (LUTS) caused by benign prostatic hyperplasia (BPH). After evaluation, benign prostatic enlargement (BPE) with benign prostatic obstruction (BPO) was evident. As treatment, the CoreTherm Concept was used in all patients with LUTS/BPO. Data parameters were retrospectively extracted and included patient age, prostate volume, energy delivery, treatment time and calculated cell kill. In addition, assessment of temperature curves and calculated intraprostatic blood flow was made to define an optimal treatment. In total, 199 treatments assessed as optimal were included in the study.
Results: There was a significant correlation between pretreatment prostate volume and energy delivery (p < .001). Age also influenced energy consumption significantly (p = .01).
Conclusion: The solid correlation between pretreatment prostate volume and age versus total energy deposition implies the recommendation that a pretreatment calculation of an appropriate energy deposition should be used in all treatments as an alternative treatment endpoint.
Introduction
Lower urinary tract symptoms (LUTS) can be caused by benign prostatic hyperplasia (BPH). Patients with LUTS/BPH and benign prostatic enlargement (BPE) with benign prostatic obstruction (BPO) can be cured either by surgical removal or microwave ablation of the obstructive prostatic tissue. High-energy transurethral microwave thermotherapy is a minimally invasive outpatient treatment option for patients with LUTS/BPO.
Microwave treatment means that a catheter, containing a microwave antenna is placed in the urethra in order to emit microwaves. The treatment catheter used in ProstaLund Feedback Treatment (PLFT, CoreTherm) contains a temperature sensor inserted into the prostate (intraprostatic sensor (IP sensor)) and is used to calculate cell kill [Citation1], which is dependent on tissue temperature and treatment time [Citation1–4]. The calculated cell kill corresponds to tissue destruction seen on transrectal ultrasound, magnetic resonance imaging and histopathology [Citation5–7]. Intraprostatic blood flow can increase considerably as a response to heat [Citation2,Citation8] and thereby act as a coolant by lowering the temperature within the prostate [Citation2,Citation3]. Intraprostatic injections of a local anesthetic containing mepivacaine and adrenaline (MA) via a catheter can abolish or minimize blood flow. The CoreTherm Concept is defined as treatment with CoreTherm in combination with intraprostatic injections of MA via an approved injection device, the Schelin catheter [Citation9,Citation10]. This treatment concept does not utilize fixed protocols. Instead, a cell kill of 20% is the recommended primary (treatment) endpoint [Citation11].
The cell kill calculation could be less accurate in two situations and is consequently to be considered unreliable. Firstly, if the IP sensor was placed incorrectly the temperature curves are defined as illogical [Citation12]. Depending on the location of the IP sensor in relation to the microwave antenna there is a risk for an over- or underestimation of the thermal dose. The energy deposition or energy delivery is the amount of energy emitted from the microwave antenna reaching the prostate adenoma, also referred to as the thermal dose. Secondly, if the intraprostatic injection of MA fails in the part of the prostate where the IP sensor is located and temperature is measured, the expected adstringent effect on the prostatic arterial supply will be lacking. A locally high blood flow will then result, and the estimated blood flow used to calculate cell kill is not representative for the rest of the gland. It would thereby be suitable if an alternative endpoint could be used in some situations, in presence of illogical temperature curves and/or a high calculated blood flow.
The primary aim of this study was to evaluate whether the desired thermal dose can be estimated from known clinical parameters, such as prostate volume and patient age, as an alternative (or secondary) endpoint when using the CoreTherm Concept.
Material and methods
Patients
After ethical approval from the Regional Ethical Review Board in Linköping (dnr 2010/394-31) we retrospectively collected baseline and treatment data from 283 consecutive treatments from one outpatient clinic. All treatments were performed during a period of six years (2003–2008). The CoreTherm Concept was used in all cases. The same urologist carried out the clinical evaluation, treatment, and follow-up in all patients with the same equipment. Evaluation of cell kill accuracy in this patient cohort has been published in a previous study [Citation11]. This study showed that the addition of MA underestimates cell kill. The responder rate was 87%, without any serious adverse events recorded in the medical journals. Five patients were treated twice, and in order to simplify the statistical analysis it was decided that the second treatment was to be included in the study.
Before treatment, all patients were evaluated regarding symptoms, voiding parameters and prostate volume. The International Prostate Symptom Score (I-PSS) or the Madsen score (interview) was used to evaluate symptoms. All patients included in the study had a score corresponding to moderate or severe symptoms. In addition, some patients were diagnosed with persistent urinary retention, and these patients received an indwelling catheter, most often because of acute urinary retention. Prostate volume was determined using transrectal ultrasound.
In order to define an optimal treatment, it was decided that three criteria had to be fulfilled. Firstly, the temperature curves had to be arranged so that the IP sensor could be considered to be in the right location. This means that logical temperature curves had to be present. Secondly, the calculated blood flow during treatment should remain at ≤20 mL/min/100 g, roughly corresponding to the blood flow in prostate glands with BPH [Citation13]. Thirdly, a calculated cell kill of ≥15% was set to be the lower limit for prostates <100 mL and ≥12% the lower limit for prostates ≥100 mL. It was thereby considered likely that the treatment went as planned, and without premature early termination.
Procedure
Prior to treatment all patients received a single-dose oral analgesic (paracetamol 1000 mg, celecoxib 200 mg), antibiotic prophylaxis (ciprofloxacin 500 mg), and a muscarinic receptor antagonist (tolterodine 4 mg). All patients had a total of 30 mL of 0.5% MA (mepivacaine 5 mg/mL, adrenaline 5 µg/mL; Carbocain adrenalin, AstraZenca AB, Södertälje, Sweden) deposited via a Schelin catheter in four quadrants of the prostate, 5 mL in the basal upper two quadrants and 10 mL in the basal lower two quadrants. Treatment was commenced immediately after infiltration to utilize the adrenaline adstringent effect, before wash out. The treatment strategy was the same in all cases, beginning with an initial effect of 30–40 Watts and gradually increasing the power aiming for a straight line temperature rise curve with a slope of approximately 45 degrees. The aim was to ablate 20% of the baseline total prostate volume according to the software calculation. If illogical temperature curves were present, treatment was paused and efforts were made to replace the IP sensor for optimization of the cell kill calculation. An indwelling catheter was left in place for 2 weeks. Patients not voiding spontaneously after this period were offered the choice between an indwelling catheter for an additional period or clean intermittent self-catheterisation.
Data analysis and statistics
Baseline data, such as patient age and prostate volume measurements, were extracted from the medical records. Concomitant diseases and ongoing medication were not consistently documented and therefore not included in the analysis. Treatment data, as total energy consumption, treatment temperature graphs and calculated blood flow during treatment, and a successful cell kill treatment goal were collected in the CoreTherm computer software. A multiple linear regression analysis was performed with prostate volume and age as independent variables and energy deposition as the dependent variable. Multiple linear regression, being used for prediction, requires the independent variables to be known beforehand. A p-value <.05 for each of the regression coefficients being different from zero was considered statistically significant. The software of Statistica version 12 (Statistica; StatSoft®, Tulsa, OK, USA) was used for all statistics.
Results
In total, 278 patients were registered for 283 consecutive treatments. Five patients were treated twice and for these only the second treatment was evaluated in the study. Logical temperature curves, indicating a correct placement of the IP-sensor, were present in 238 treatments. Furthermore, in 215 treatments a calculated blood flow of ≤20 mL/min/100g was evident. Finally, an acceptable cell kill, as defined in the study, was achieved in 234 treatments. The statistical analysis included all treatments judged as optimal, in total 199 (72%) of 278 treatments ().
Table 1. Assessment of treatments in order to define an optimal treatment in the study.
Baseline parameters of patient age and prostate volume, as well as the treatment parameters treatment time, energy deposition and cell kill are reported in . As these data parameters were not normally distributed, each variable is presented with values for median, range, lower and upper quartile. As can be seen in the table, one quarter of the patients were 75 years or older and one quarter of the patients had prostates of 87 mL or larger.
Table 2. Baseline and treatment characteristics of the study population.
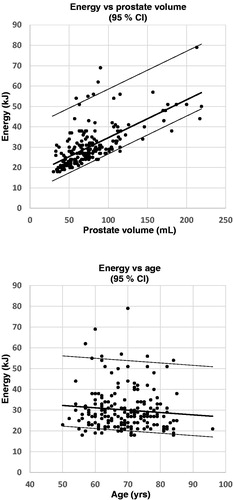
On multiple linear regression analysis both prostate volume and patient age were significant predictors of thermal dose. The magnitude of the individual influence and impact of prostate volume and age on the energy consumption can be derived from the equation that is the result of the multiple linear regression analysis: [thermal dose (kJ) = 27 + 0.19 × prostate volume (mL) − 0.16 × age (years)]. The regression analysis shows that the impact on the thermal dose is more affected by prostate volume (0.19 kJ/mL) as compared to age (0.16 kJ/year). The equation shows were the regression line cuts the y-axis (intercept) and that there are two independent variables that affects the thermal dose independently, but not to the same extent. The thermal dose increased with increasing prostate volume (p < .001) and decreased with increasing age (p = .01), .
Figure 1 a and b. The figures are derived from the multiple linear regression analysis of baseline prostate volume (1a (upper figure), r = 0.98) and age (1b (lower figure), r = 0.14) as the independent variables versus energy delivery as the dependent variable. The broad solid middle line represents the regression line and the thin solid lines on each side represents the upper and lower 95% confidence interval. All cases judged as optimal were included in the analysis and each case is represented by a black dot (n = 199).
Discussion
This study shows a correlation between both baseline prostate volume and age versus total energy delivery. The correlation between baseline prostate volume versus energy delivery was a logical and anticipated outcome. The negative correlation between age and thermal dose was also an expected finding. It is important to state that prostate volume and age independently affect the required thermal dose, but not to the same extent.
To our knowledge this is the first study that has shown a connection between pretreatment prostate volume and age versus energy delivery. The first clinical study that used intraprostatic injections of MA via the Schelin catheter showed that the thermal dose was significantly lower in those patients receiving injections of MA, compared to previously treated patients without MA [Citation9]. In this study by Schelin the mean thermal dose was 65 kJ in the patients receiving MA, compared to 172 kJ in those not receiving MA. The calculated blood flow and treatment time were also significantly reduced in the group receiving injections.
The difference in thermal dose between patients receiving MA in the study by Schelin (mean 65 kJ) compared to our study (median 28 kJ) can possibly be explained by differences in injection technique, using three locations instead of mainly four in our study. The fact that intraprostatic injections of MA so clearly reduce the thermal dose, treatment time and calculated blood flow is an indicator that the most counterproductive factor for heat build-up is blood flow.
If the blood flow is reduced, or abolished, all that is left, in an ideal situation, is a devascularized prostate. It is reasonable to assume that a larger prostate needs more energy delivered in order to achieve the same percentage of volume reduction, compared to a smaller prostate. In the study by Knutson et al. the addition of MA when using PLFT/CoreTherm also confirmed reduced treatment time and energy delivery [Citation14].
In a preclinical study by Bolmsjö [Citation1], two important statements were made. Firstly, only by directly monitoring the intraprostatic temperature during treatment and adjusting microwave power accordingly was it possible to achieve a sufficiently high temperature resulting in cell kill. Secondly, during treatment, it was important to avoid interruptions of power, as this can reduce cell kill substantially, also making the cell kill calculation unreliable. To trust the real time calculation of cell kill, the temperature curves must be considered logical, and the avoidance of interruptions in temperature rise is essential.
A correct positioning of the intraprostatic sensor must be assured, as well as an actual reduced or minimized blood flow when MA is used. The IP sensor registers temperatures at three locations within the prostatic adenoma during treatment. The temperatures are displayed as three temperature curves on a monitor in real time during the procedure. When placement of the IP sensor is correct, the temperature curves will be considered reliable and subsequently referred to as logical. The presence of logical temperature curves is a basis for a correctly calculated cell kill by the computer software [Citation12].
Thus, during treatment, when illogical temperature curves are present and/or a high blood flow is monitored, there is an apparent need for an alternative solution, other than repositioning of the IP sensor or termination of treatment. In clinical practice, recommendations have evolved during the continuous development of the treatment concept and are provided during the educational phase and presented in the instructions for use (IFU, ProstaLund AB, Lund, Sweden). In the IFU the use of a primary and secondary endpoint is recommended. The calculation of the secondary endpoint (or energy points in IFU) is based on clinical experience and, in part, previous preliminary data regarding the relationship between prostate volume and energy delivery. In clinical practice the required thermal dose based on prostate volume is calculated before treatment, according to the IFU.
The findings in our study show that the use of a thermal dose calculation before treatment is possible. Our findings clearly support this approach, regardless of nomenclature used (thermal dose, secondary endpoint, alternative endpoint, energy points). A primary endpoint of 20% in combination with a secondary endpoint, based on a thermal dose calculation, should therefore be used in all cases. Most importantly, when using the CoreTherm Concept in patients with LUTS/BPO it is possible to use a calculation of thermal dose as a safety parameter to avoid the deposition of excessive energy.
Our analysis was based on treatments considered optimal in all aspects. Based on the inclusion criteria 28% of our treatments were excluded from the statistical analysis.
Although no serious adverse events were found in the medical records, it is conceivable that over- or undertreatment may occur mainly among these 28% of the patients.
In this study, the calculated cell kill primary treatment endpoint works, with reliable treatment parameters and guidance for when to finish treatment, in 72% of the cases. In the other 28% an alternative treatment endpoint is needed to ensure a safe and effective procedure.
One quarter of the patients in the study were ≥75 years of age and another quarter had prostates ≥87 mL, indicating that the CoreTherm Concept can be used in considerable proportion of patients, regardless of age and prostate size, reducing the need for invasive surgical procedures or other non-office-based treatments.
The strength of this study is that our analyses are based on a large sample of consecutive patients who underwent a standardized procedure by the same urologist using the same equipment. The weaknesses of this study were the retrospective design and lack of baseline patient data that could have been further analyzed. There is a possibility that other baseline factors related to tissue composition, comorbidity or medications could be used in order to fine-tune the thermal dose. A detailed medical history of cardiovascular disease, diabetes mellitus, and patient medication that could eventually affect prostatic blood flow would perhaps have strengthened the definition of the study population.
Conclusion
The solid correlation between pretreatment prostate volume and age versus total energy deposition implies the recommendation that a pretreatment calculation of an appropriate energy deposition should be used in all treatments as an alternative treatment endpoint.
Acknowledgements
We would like to thank Sonny Schelin, M.D., Ph.D. for his support and advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Bolmsjö M, Schelin S, Wagrell L, et al. Cell kill modeling of microwave thermotherapy for treatment of benign prostatic hyperplasia. J Endourol. 2000;14(8):627–635.
- Wagrell L, Schelin S, Bolmsjö M, et al. Intraprostatic temperature monitoring during transurethral microwave thermotherapy for the treatment of benign prostatic hyperplasia. J Urol. 1998;159(5):1583–1587.
- Bolmsjö M, Sturesson C, Wagrell L, et al. Optimizing transurethral microwave thermotherapy: a model for studying power, blood flow, temperature variations and tissue destruction. Br J Urol. 1998;81(6):811–816.
- Bhowmick P, Coad JE, Bhowmick S, et al. In vitro assessment of the efficacy of thermal therapy in human benign prostatic hyperplasia. Int J Hyperthermia. 2004;20(4):421–439.
- Hoffman AL, Laguna MP, de la Rosette JJ, et al. Quantification of prostate shrinkage after microwave thermotherapy: a comparison of calculated cell-kill versus 3D transrectal ultrasound planimetry. Eur Urol. 2003;43(2):181–187.
- Huidobro C, Bolmsjö M, Larson T, et al. Evaluation of microwave thermotherapy with histopathology, magnetic resonance imaging and temperature mapping. J Urol. 2004;171(2):672–678.
- Vesely S, Muller M, Knutson T, et al. Transurethral microwave thermotherapy of the prostate – evaluation with MRI and analysis of parameters relevant to outcome. Scand J Urol Nephrol. 2008;42(1):53–58.
- Wagrell L, Sundin A, Norlen B. Intraprostatic blood-flow changes during ProstaLund feedback treatment measured by positron emission tomography. J Endourol. 2005;19(7):873–877.
- Schelin S. Mediating transurethral microwave thermotherapy by intraprostatic and periprostatic injections of mepivacaine epinephrine: effects on treatment time, energy consumption, and patient comfort. J Endourol. 2002;16(2):117–121.
- Schelin S, Claezon A, Sundin A, et al. Effects of intraprostatic and periprostatic injections of mepivacaine epinephrine on intraprostatic blood flow during transurethral microwave thermotherapy: correlation with [15O]H2O-PET. J Endourol. 2004;18(10):965–970.
- Stenmark F, Brudin L, Stranne J, et al. High-energy feedback microwave thermotherapy and intraprostatic injections of mepivacaine and adrenaline: an evaluation of calculated cell kill accuracy and responder rate. Scand J Urol. 2014;48(4):374–378.
- Schelin S. Development of feedback microwave thermotherapy in symptomatic benign prostatic hyperplasia. Thesis. Lund University Hospital, Sweden; 2006.
- Inaba T. Quantitative measurements of prostatic blood flow and blood volume by positron emission tomography. J Urol. 1992;148(5 Part 1):1457–1460.
- Knutson T, Johansson A, Damber JE, et al. Intraurethral prostate injections with mepivacaine epinephrine: effects on patient comfort, treatment time and energy consumption during high-energy transurethral microwave thermotherapy. Scand J Urol Nephrol. 2009;43(4):300–306.
