Abstract
Purpose
The extent of late side-effects in prostate cancer patients, after radical prostatectomy (RP = reference group) and salvage radiation therapy (SRT) in a self-reporting perspective (PROM) is still under-reported. We aimed to investigate the rate and severity of side-effects and quality-of-life (QoL) according to PROM.
Methods and materials
A PROM survey was administered to a cohort of SRT patients matched to a reference group with median follow-up 10 years after surgery. In total, 740 patients were analyzed. To investigate the association between SRT versus reference group regarding side-effects and QoL, a Poisson regression analysis was conducted and presented as relative risk estimates (RR) together with 95% confidence intervals regarding questions related to urinary, rectal, sexual symptoms and QoL.
Results
RRs ranged from of 1.7–6.5 on rectal symptoms and 1.2–1.4 for urinary symptoms. In general health, QoL and sexual function all RRs were below 1.1. With increasing age, higher RRs were seen for urinary leakage and lowered sexual function whereas longer time following irradiation showed higher RRs for rectal symptoms and rectal leakage. Limitations of this study include the cross-sectional design and lack of baseline assessment.
Conclusions
Adding SRT to RP does not seem to result in other than acceptable side-effects in the majority of men receiving SRT when taking a long follow-up time (median 10 years after surgery) into account. However, a subset of men develop severe side-effects where rectal bleeding dominates.
Introduction
Although multiple randomized studies have shown the benefit of adjuvant radiotherapy (ART) after radical prostatectomy (RP) [Citation1–3] many centers prefer to wait until the disease relapse and then administer salvage radiation therapy (SRT). Recently the result of three randomized studies (Raves, Radicals and GETUG-AFU 17; Clinical trial identification: RADICALS: ISRCTN40814031 GETUG-17: NCT00667069 RAVES: NCT00860652), comparing early SRT to ART, has been orally presented (Abstract ESMO 2019) and they concluded that ART entails 50% overtreatment of patients. They also show that the ART is associated with a higher extent of urinary and bowel complications than early SRT, without any improvement in progression-free survival at 5 years of follow-up. These findings, favoring SRT, stresses the wait-and-see approach, monitoring the PSA (Prostate-specific antigen) after RP. If relapse of the disease occurs, (PSA levels >0.2 ng/ml), the choice would be SRT and this occurs in > 30% of men who undergo RP internationally [Citation4,Citation5]. In Sweden, these results are similar according to the National Prostate Cancer Register (www.NPCR.se). SRT offers the patient a second possibility to be cured when the disease is relapsing, however, understanding which group of patients that would benefit the most is still unclear as, according to Bartkowiak et al. [Citation6], at least 50% of patients will not benefit from SRT. However, a continued increase is probably to be expected as many more patients with high-risk disease are accepted for surgery today [Citation7].
The large number of men undergoing SRT in combination with the rather high risk of over-treatment requires us to carefully map the risk of side-effects and associated influence on quality-of-life (QoL). Well known side-effects after SRT include erectile dysfunction; urinary symptoms such as incontinence, irritable bladder and hematuria; and bowel dysfunction including bleeding and incontinence. In general, however, SRT is considered to be associated with few severe side-effects and is well accepted by patients [Citation8]. Many publications are from large, highly specialized units and lack long-term follow-up so there is a need to bring further clarity on the extent of side-effects [Citation9].
In assessing the side-effects after radiation therapy for prostate cancer many studies have reported outcomes according to scales and criteria posed in the Radiotherapy Oncology Treatment Group/Common Toxicity Criteria (RTOG/CTC)[Citation10,Citation11]. The reliability and objectiveness of these records has been questioned [Citation12,Citation13]. Instead, opinions directly from the patients, patient-reported outcome measures (PROM), are considered more subjective and reliable in the assessment of side-effects and QoL.
The primary aim of this study was to investigate the rate and severity of side-effects as well as QoL according to PROM in patients treated with SRT after RP and comparing these with a group of patients who underwent only RP in a population from a defined catchment area with a median follow-up of 10 years. Secondary aims included investigating the associations between side-effects and age as well as length of follow-up time and Planning Target Volume (PTV).
Patients and methods
Patients
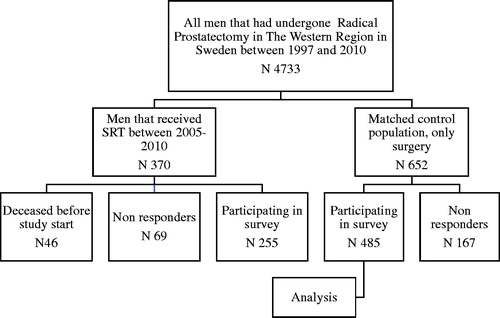
All patients who underwent SRT (n = 370) for prostate cancer at the Sahlgens University Hospital (catchment area of ∼ 1.6 million inhabitants) during 2005–2010 were eligible for this follow-up study of post-radiation morbidity. Men who were still alive (n = 324) were asked to complete a PROM-survey sent by postal mail during 2015. We checked the prevalence of post-radiation morbidity by analyzing medical records among those men who did not respond to this survey or had died before invitation. We identified a reference group from the NPCR comprising men who had undergone prostatectomy only. This reference group was matched according to age, year of surgery, and hospital. Each SRT patient was matched with two patients who had undergone RP only (). Both the SRT and the reference group had undergone surgery during the years 1997 through 2010 in the Western Region of Sweden. Three of the men in the control group appeared to have undergone SRT after 2010 and were thus excluded, resulting in a total of 652 men who received the same survey. For both groups, a reminder was sent if no response was received after 1 month. Clinical data such as pT-stage were retrieved from NPCR (). We collected information from medical records on severe urinary tract and rectum complications on those patients who did not complete the survey and deceased men. A severe adverse complication was defined as a complication requiring hospitalization or surgical intervention.
Table 1. Patient and treatment characteristics.
Radiation
The SRT was delivered as 2 Gray (Gy) per fraction 35 times, resulting in a total dose of 70 Gy by a Clinical iX System Linear Accelerator (Varian Medical Systems, Palo Alto, CA). The radiation therapy was delivered as a 3-field conformal technique using 15 MV photons (n = 248) or as a 7-field intensity-modulated radiation therapy technique (IMRT) using 6 MV photons (n = 7). Neither of the SRT patients were treated with Androgen Deprivation Therapy (ADT) concomitant along with the radiation therapy. Nor did any patient receive an extended radiation field against lymph nodes. The volume of the PTVs were extracted from the EclipseTM treatment planning system (Varian Medical Systems).
Instruments
A Health Declaration Form (HDF) was administered together with the survey and enabled us to evaluate the current health status and medication. The survey used has frequently been administered within the prostate cancer care in Sweden and was first described in 1994 (QUFW94), and has undergone some changes over the years [Citation14,Citation15]. From this survey, based upon 53 questions, we selected 14 questions to assess possible differences between irradiated patients and the reference group (in Supplementary Appendix) as (RR) estimates. The domains chosen were those mirroring the most frequently reported side-effects associated with prostate cancer treatment, e.g. urinary incontinence and other urinary symptoms (questions 13, 15, 18, 23 in Supplementary Appendix); bowel symptoms, including discharge of blood (questions 24, 26, 28, 35 in Supplementary Appendix); sexual function (questions 37, 38, 43, 44 in Supplementary Appendix); and health-related QoL (questions 9, 10 in Supplementary Appendix). When analysing the responses to these questions, we dichotomised the scores (in Supplementary Appendix). For those responses where the 95% CI (confidence intervals) for RR were above 1, we analyzed the intensity of symptoms and QoL responses in addition.
Statistical analysis
RRs between the SRT and reference groups were calculated using Poisson regression. Corresponding 95% CIs were calculated using robust error variances. The effect of age, PTV, and length of follow-up (time lapse between radiation therapy and survey) to the responses was investigated in the SRT group. In this analysis, age, PTV, and the time to follow-up were dichotomized at their median values.
The proportion of missing responses to the different questions was calculated, and each question was analyzed based on the available answers.
The statistical analyses were performed in R Statistical Software (Version 3.5.0) and SPSS.
Ethics
The Research Ethics Board approved the study, (EPN 488-13).
Results
The response rate was 79% (n = 255) in the SRT group and 74% (n = 485) in the reference group. However, the response rate varied between the different questions (). Nevertheless, almost all of those who responded to the survey also responded to the HDF (). The median time from surgery to survey was 10 years in both groups and median time from radiation therapy to survey was 6.7 years (). A considerable difference was found in the rate of ADT medication between the two groups with 22% in the SRT group compared to 6% in the reference group ().
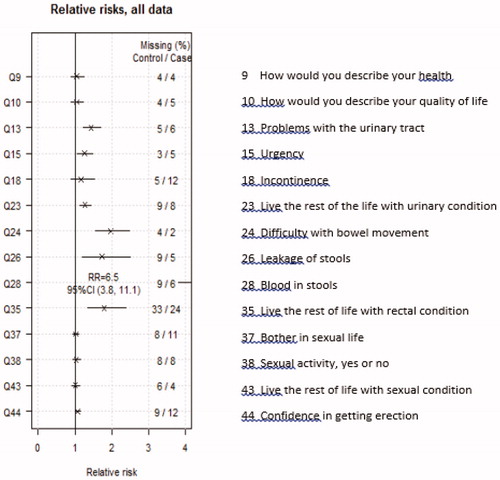
Figure 2. Relative risk ratios and 95% confidence intervals for the risk of side-effects SRT versus reference group.
Comparison between groups
The impact of radiation was largest on the rectal symptoms, with RRs ranging from 1.7 to 6.5. The impact on urinary symptoms was less, with RRs between 1.2–1.4. For general health, QoL, or sexual function the impact was even less, where all RRs were below 1.1 () and the response to the question on sexual activeness (Question 38) was 30, respectively, 32% in the SRT versus the reference group. When comparing the groups according to use of ADT and the influence it would bring to the responses in the survey we found small differences between the SRT group and the reference group among those with ADT and those without ADT (see figure in Supplementary Appendix). If anything, use of ADT seemed to decrease the difference in bowel symptoms between the two groups.
In general, the intensity of symptoms was low in both the SRT and the reference group. Prevalence figures for the groups are presented in .
Figure 3. The intensity of symptoms and quality-of-life assessments when significant differences between SRT and reference were found. Percentage of total on column. Blue = SRT; Red = reference group. ‘1’ not at all, ‘2’ a little, ‘3’ to some extent, ‘4’’ very much.
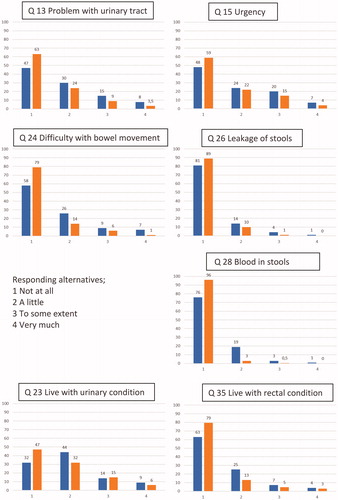
The proportion of men who reported at least one “severe symptom” (as classified by responding 4/5) to one of the selected questions (13, 15, 18, 24, 26, 28 in Supplementary Appendix) was 24% in the SRT group and 11% in the reference group. In the SRT group, 16% reported at least one severe urinary symptom and 8% at least one severe rectal symptom, compared to 9% and 2% in the reference group, respectively (results not shown in ).
Urinary, rectal and sexual side-effects – only SRT group
RRs for age at radiation and length of follow-up time after radiation are presented in . With higher age, higher RRs were seen for urinary leakage and sexual activity, with CIs above 1, while for length of time after radiation, higher RRs were seen with rectal symptoms, predominantly rectal leakage, and bother from rectal function. Rectal bleeding was becoming less frequent with follow-up (). For length of time between surgery and radiation a small, but positive effect on QoL and incontinence was seen, with RRs close to 0.9 (figure not presented).
Figure 4. (a) Relative risk, age at surgery, dichotomized at its median, SRT; (b) Relative risk, time to survey, dichotomized at its median, SRT; (c) Relative risk ratios and 95% confidence intervals for the risk of side-effects, PTV versus scores, SRT.
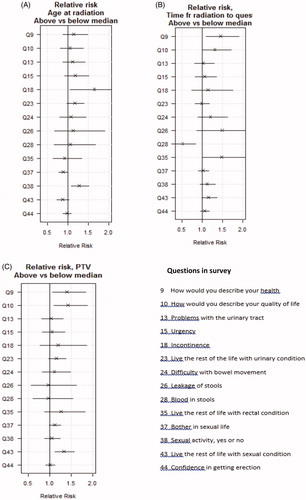
Larger PTV size was associated with worse self-assessed general health and overall QoL and to some extent with bother about sexual function ().
Analysis of non-responders and deceased
Altogether, 236 patients did not respond to the survey (69 in the SRT group and 167 in the reference group). In the SRT group, four men were described within their medical records as having severe side-effects; two with rectal and urinary bleeding, requiring treatment, one with rectal incontinence and one who received deviation surgery for anastomosis stricture. Based on medical records, none of the 167 men were found to have had severe side-effects within the reference group.
Forty-six men in the SRT group were deceased at the time of the survey. About half of them died of prostate cancer, four from cardiovascular disease and the remaining men from other malignancies. The relationship between radiation and urinary tract and rectal symptoms in this group was often difficult to assess as symptoms may stem from malignancies other than the treated prostate cancer. However, three men had severe urinary incontinence requiring surgery (AMS 800) and one patient had fecal incontinence.
Discussion
This study aimed to show additional comorbidity when comparing patients receiving SRT after prostatectomy to patients in the group that had gone through RP only. The SRT group more frequently reported symptoms from the rectum, but also, to less extent, from the urinary tract. We found no differences in sexual function or global QoL.
The primary finding when comparing these two groups was rectal symptoms, predominantly blood in stools. In the SRT group, 23% of the men reported rectal bleeding compared to 3% in the reference group. However, the frequency of severe rectal bleeding was low; 11 out of 239 patients (5%) reported grade 3–4 (one patient receiving ongoing treatment with warfarin). The prevalence of fecal leakage of any severity was lower, yet 5% reported grade 3–4 in the SRT group compared to 1% in the reference group. Our results are in agreement with Cortes-Gonzales et al. [Citation16] and Alsadius et al. [Citation17] (PROM data) that bleeding and bowel inconveniences were dominant side-effects after radiation therapy in patients with prostate cancer.
Reporting side-effects after radiation therapy is rarely based upon PROM data since most publications have been based upon doctor-reported toxicity. In one overview by Raziee and Berlin [Citation18] from 2016, only seven studies have reported side-effects from PROM data.
Side-effects may appear many years after radiation therapy demanding very long-follow-up for accurate evaluation of toxicity [Citation19]. In our study, with very long follow-up, we found a small increase in fecal incontinence with longer follow-up, RR = 1.1 (95% CI = 1.0–1.3) which might be at least partly explained by increasing age [Citation20,Citation21]. On the contrary, we observed that rectal bleeding diminished with time, which also has been reported by others previously [Citation17,Citation22,Citation23]. On the other hand, Berlin et al. [Citation24] found, following patients that had undergone postoperative radiation therapy, that the side-effects (rectal and urinary) were stable for at least 5 years in follow-up.
In this study, we found no obvious relation between PTV and symptoms reported but we have not calculated Dose Volume Histograms (DVH) or absorbed dose to the rectum. Surprisingly, even though the PTV magnitudes were not associated with rectal or urinary side-effects, they were linked to worse QoL; the larger PTV the worse QoL and global health. Also, in our data, sexual bother was affected by the PTV size. However, the data within this study do not enable us to identify the factors underlying these results and demands further studies, preferably from analysing DVH and absorbed dose to organs at risk and a study on this is planned. Newer treatment techniques, as IMRT or VMAT (Volumetric Arc Technique) could decrease the dose to the rectum; this has not yet been shown [Citation25].
Urinary symptoms were frequently reported in both the SRT and the reference group, as 9% of men in the reference group reported severe urinary symptoms (grade 4 or 5) compared to 16% of men in the SRT group. The RR of reporting “problems with the urinary tract” (question 13 in Supplementary Appendix) was 1.4 (95% CI = 1.2–1.7) in the SRT group compared to the reference group. The impact on urge and incontinence was lower, RRs = 1.3 (95% CI = 1.1–1.5) and 1.2 (95% CI = 0.9–1.5), respectively. For incontinence, a similar result was reported by Son et al. [Citation26].
In our study, the time between surgery and SRT varied between 0.25 and 12.75 years, but the effect on urinary symptoms with a longer time interval between surgery and radiation was negligible with all RRs ∼ 1. Instead, a positive effect on global QoL and health was shown with a longer time interval between surgery and SRT, perhaps due to the positive reaction on an improvement of urinary leakage and a non-relapsing PSA.
Our study also included four questions regarding sexual function. We found no results indicating that SRT deteriorated sexual function. However, this group of men are considered to already have a poor sexual function as a result of surgery, as documented by others [Citation27,Citation28]. Surprisingly, the use of ADT did not seem to worsen the side-effects or QoL in our study, in contrast to other authors’ [Citation29,Citation30].
This study has several strengths and limitations. One strength is that men in this study represent an unselected population. Our population represented all men within a catchment area who received SRT during a defined time period. All men except 8% in the SRT and 15% in the reference group were possible to trace. Another strength is the use of a validated PROM used in many thousands of patients in the national registry NPCR. This study also had a high survey response rate of 74%. We obtained further information about the health status and current medications via an HDF, also attached within the survey. This resulted in further knowledge and facilitated an understanding of the results, for instance revealing that at the time of taking the survey, a considerably greater pproportion of the men were prescribed ADT in the SRT group.
Limitations of this study are its cross-sectional and single institution character and that no baseline assessment was available. Hence, a matching procedure was performed in order to make the reference and SRT groups comparable.
Conclusion
To summarize, adding SRT to RP did not result in side-effects very different from the men in the RP only group, in the majority of men receiving SRT, when taking a long follow-up time (median 10 years after surgery) into account. However, a subset of men develop severe side-effects and there is a challenge to develop a risk assessment tool in the future, to identify these men. The decision of SRT or not should, consequently, be based upon careful consideration of pros and cons when advising the patient.
Disclosure statement
The authors have no conflict of interest to declare.
References
- Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243–250.
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–2027.
- Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190(2):441–449.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–439.
- Freedland SJ, Vidal AC, Howard LE, et al. Race and risk of metastases and survival after radical prostatectomy: results from the SEARCH database. Cancer. 2017;123(21):4199–4206.
- Bartkowiak D, Thamm R, Bottke D, et al. Prostate-specific antigen after salvage radiotherapy for postprostatectomy biochemical recurrence predicts long-term outcome including overall survival. Acta Oncol. 2018;57(3):362–367.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629.
- Feng M, Hanlon AL, Pisansky TM, et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(5):1417–1423.
- Wallis CJD, Glaser A, Hu JC, et al. Survival and complications following surgery and radiation for localized prostate cancer: an international collaborative review. Eur Urol. 2018;73(1):11–20.
- Nath SK, Sandhu AP, Rose BS, et al. Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(2):435–441.
- Katayama S, Striecker T, Kessel K, et al. Hypofractionated IMRT of the prostate bed after radical prostatectomy: acute toxicity in the PRIAMOS-1 trial. Int J Radiat Oncol Biol Phys. 2014;90(4):926–933.
- Wilson KA, Dowling AJ, Abdolell M, et al. Perception of quality of life by patients, partners and treating physicians. Qual Life Res. 2000;9(9):1041–1052.
- Sonn GA, Sadetsky N, Presti JC, et al. Differing perceptions of quality of life in patients with prostate cancer and their doctors. J Urol. 2013;189(1 Suppl):S59–S65.
- Widmark A, Fransson P, Tavelin B. Self-assessment questionnaire for evaluating urinary and intestinal late side effects after pelvic radiotherapy in patients with prostate cancer compared with an age-matched control population. Cancer. 1994;74(9):2520–2532.
- Fransson P, Tavelin B, Widmark A. Reliability and responsiveness of a prostate cancer questionnaire for radiotherapy-induced side effects. Support Care Cancer. 2001;9(3):187–198.
- Cortes-Gonzalez JR, Castellanos E, Sandberg K, et al. Early salvage radiation therapy combined with short-term hormonal therapy in recurrent prostate cancer after radical prostatectomy: single-institution 4-year data on outcome, toxicity, health-related quality of life and co-morbidities from 184 consecutive patients treated with 70 Gy. Int J Oncol. 2013;42(1):109–117.
- Alsadius D, Olsson C, Pettersson N, et al. Patient-reported gastrointestinal symptoms among long-term survivors after radiation therapy for prostate cancer. Radiother Oncol. 2014;112(2):237–243.
- Raziee H, Berlin A. Gaps between Evidence and practice in postoperative radiotherapy for prostate cancer: focus on toxicities and the effects on health-related quality of life. Front Oncol. 2016;6:70.
- Goenka A, Magsanoc JM, Pei X, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol. 2011;60(6):1142–1148.
- Leufgens F, Berneking V, Vogeli TA, et al. Quality of life changes >10 years following postoperative radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 2019;105(2):382–388.
- Kopp RP, Marshall LM, Wang PY, et al. The burden of urinary incontinence and urinary bother among elderly prostate cancer survivors. Eur Urol. 2013;64(4):672–679.
- Lee JY, Daignault-Newton S, Heath G, et al. Multinational prospective study of patient-reported outcomes after prostate radiation therapy: detailed assessment of rectal bleeding. Int J Radiat Oncol Biol Phys. 2016;96(4):770–777.
- Thor M, Apte A, Deasy JO, et al. Dose/volume-response relations for rectal morbidity using planned and simulated motion-inclusive dose distributions. Radiother Oncol. 2013;109(3):388–393.
- Berlin A, Cho E, Kong V, et al. Phase 2 trial of guideline-based postoperative image guided intensity modulated radiation therapy for prostate cancer: toxicity, biochemical, and patient-reported health-related quality-of-life outcomes. Pract Radiat Oncol. 2015;5(5):e473–e482.
- Bruner DW, Hunt D, Michalski JM, et al. Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer. 2015;121(14):2422–2430.
- Son CH, Melotek JM, Liao C, et al. Bladder dose-volume parameters are associated with urinary incontinence after postoperative intensity modulated radiation therapy for prostate cancer. Pract Radiat Oncol. 2016;6(5):e179–e185.
- Moinpour CM, Hayden KA, Unger JM, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26(1):112–120.
- Cremers RG, van Lin EN, Gerrits WL, et al. Efficacy and tolerance of salvage radiotherapy after radical prostatectomy, with emphasis on high-risk patients suited for adjuvant radiotherapy. Radiother Oncol. 2010;97(3):467–473.
- Lubeck DP, Grossfeld GD, Carroll PR. The effect of androgen deprivation therapy on health-related quality of life in men with prostate cancer. Urology. 2001;58(2 Suppl 1):94–100.
- Bylow K, Dale W, Mustian K, et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology. 2008;72(2):422–427.