Abstract
Objective
To assess the value of a first MRI examination and image-fusion-guided biopsies in men with low-risk prostate cancer who have been on active surveillance (AS) for several years with no signs of progression.
Patients and Methods
All 45 participants from two centers who had not previously had an MRI were included. They had been on AS for T1c Gleason score 6 prostate cancer for 2.6 to 6.7 years and had 2 to 5 sets of systematic biopsies with a total of 1640 cores. All underwent a bi-parametric MRI, PI-RADS ≥ 3 lesions were targeted with image-fusion-guided biopsies. Primary outcome measure: detection of Gleason score ≥7 cancer.
Results
Twenty-five of the 45 men (56%) had a total of 30 suspicious MRI lesions. The lesion with the highest score was a PI-RADS 3 in 18, a PI-RADS 4 in 5 and PI-RADS 5 in 3 men. Targeted biopsies from the 30 lesions detected Gleason score 7 cancer in 6 men. Of these six cancers, four were located in the apical and one in the anterior/apical part of the prostate. A Gleason score 7 cancer was detected in 3 of 5 men with PSA density >0.15 ng/ml/cm3.
Conclusions
Even after several years of AS with stable PSA values and many sets of systematic biopsies, a first MRI and targeted biopsies lead to the detection of Gleason score 7 (ISUP 2 and ISUP 3) cancer in a significant proportion of men, particularly among those with a high PSA density.
Introduction
Active surveillance (AS) in men with low-risk prostate cancer is an accepted alternative to immediate treatment, with excellent long-term outcome according to prospective trials with up to 15 years of follow-up [Citation1]. The use of magnetic resonance imaging (MRI) and targeted biopsies leads to the detection of high-grade cancer in a substantial proportion of men with low-grade cancer on systematic biopsies and is now recommended in the European guidelines to select men for AS [Citation2]. There are, however, many men on AS who have not had an MRI because they were diagnosed before a ‘selection MRI’ was recommended. It is reasonable to offer these men a MRI if their PSA values are rising, but the need for a first MRI in men with a stable PSA level is not known. The yield of repeat systematic biopsies is low in men who have been on AS several years [Citation3], but as usually only the posterior parts of the prostate are sampled some men may have an undetected high-grade cancer in the anterior parts of the gland despite negative systematic repeat biopsies [Citation4,Citation5]. We therefore investigated the diagnostic yield of a first MRI and targeted biopsies in men that have been on active surveillance with stable PSA values for at least 2.5 years and undergone at least two sets of systematic biopsies while under active surveillance.
Patients and methods
Patients
This study included men on active surveillance within the observational part (ObsQoL) of the prospective multicenter trial Study of Active Monitoring in Sweden (SAMS) [Citation6]. SAMS-ObsQoL included men aged 40 to 75 years with low- or intermediate-risk prostate cancer diagnosed the past 6 months. The trial started recruiting in 2011. The SAMS-ObsQoL protocol specified surveillance as PSA testing every six months, annual digital rectal examination, and a repeat 8- to 12-core systematic transrectal biopsy every second year. MRI has never been recommended in the SAMS protocol, but as MRI was increasingly used for prostate cancer diagnostics a box was added to the clinical report form in 2014 to register use of MRI in men participating in the trial.
The present study included all 45 men included in SAMS-ObsQoL at Karolinska University Hospital Solna and Farsta Urology Clinic who had a T1c Gleason score 3 + 3 = 6 cancer that had not been upgraded on repeat biopsy, and who had not previously had a prostate MRI. The men were contacted by letter and offered an MRI and, if any suspicious lesion would be found, subsequent targeted biopsies. Those who did not respond to the letter were contacted by phone. All 45 men accepted and were included in the analysis. The SAMS trial, including the present study, was approved by the Regional Ethical Review Board at Lund University (EPN 2010/598 with amendment in 2016).
Magnetic resonance imaging
MRI was performed at three different sites using a bi-parametric protocol including T2-weighted imaging in three orthogonal planes (sagittal, axial and coronal), axial T1-weighted covering the small pelvis and diffusion-weighted imaging (DWI), with a calculated high b-value and an ADC-map 16 patients were scanned on a Magnetom Aera 1,5 T (Siemens Medical Systems, Erlangen, Germany), eight patients on a Magnetom Verio 3 T (Siemens Medical Systems, Erlangen, Germany) and 21 patients on an Achieva 3 T, (Philips, Einthoven, Holland). The MRI protocol is briefly described in Supplement 1. The participants were asked no to ejaculate the 3 days before the examination. On the day of the MRI, they were recommended to use a small enema approximately 2 h before the examination. Just before scanning, an intramuscular injection of either 20 mg of Buscopan or 1 mg Glucagone was given.
MRI assessment
One radiologist with 6 years’ experience of prostate MRI reading assessed all examinations in the study. Prostate volume was calculated from measurements of maximum height (superior/inferior) and depth (anterior/posterior) on sagittal images, and width (right/left) on axial images, using the formula H × D×W × 0.52. Assessments were done according to PI-RADS v2 with the exemption of dynamic contrast enhancement (DCE) given in the PI-RADS document under ‘Assessment without adequate DCE’. All lesions were depicted in the Swedish national prostate template along with size and zonal location. When a lesion suspicious for malignancy was found, the boundaries of the prostate and the lesion were outlined in the radiology software for the BioJet system (D&K Technologies GmbH, Barum, Germany).
Targeted biopsies
All patients’ urine was tested with dipslide to exclude bacteriuria. One tablet of Ciprofloxacin 750 mg was given orally as antibiotic profylaxis. Transrectal ultrasound-guided targeted biopsies of the lesions were taken in a urology out-patient clinic by one experienced urologist using the BioJet image-fusion system and 18-gauge core needles with a spring-loaded biopsy gun. At least two (median 3) targeted biopsies were taken from all PI-RADS 3–5 lesions after local anesthesia with 5–10 mL of 2% lidocaine given via a 22-gauge spinal needle. No systematic biopsies were taken.
Histology from targeted biopsies
Targeted biopsy cores were separately potted, formalin fixed in separate containers and graded by experienced uropathologists according to International Society of Urological Pathology 2014 [Citation7].
Outcome measures and statistical analysis
The primary outcome measure was upgrading to Gleason score ≥ 7 on the targeted biopsy. Secondary outcome measures were the influences of PSA density and PI-RADS score on Gleason score ≥ 7 cancer detection. The 95% confidence intervals (CIs) were calculated for proportions according to the binomial distribution. Proportions were compared with Fisher’s exact test.
Results
All 45 patients had a T1c Gleason score 3 + 3 = 6 prostate cancer. Their average age at inclusion in the present study was 66.4 years (range 55 to 76 years). They had been on active surveillance for 2.6 to 6.7 years (median 3.5 years) and had 2 to 5 (median 3.0) sets of systematic biopsies, with a total of 1640 of biopsy cores while on surveillance (). None of them had previously had an MRI of the prostate.
Table 1. Patients characteristics for all the included men.
MRI showed a total of 30 PI-RADS ≥ 3 lesions in 25 of the 45 men (56%). The lesion with the highest score was a PI-RADS 3 in 18, a PI-RADS 4 in 4 and PI-RADS 5 in 3 men (). A total of 101 targeted biopsies from these lesions detected Gleason score 7 (ISUP 2 and ISUP 3) cancer in 6 men, 2 in the 17 PI-RADS 3 lesions and 4 in the 8 PI-RADS 4-5 lesions (). These six men represent 13% of all 45 men (95% CI 5-27%) and 24% of the men with a lesion (95% CI: 9-45%). Of the 6 Gleason score ≥ 7 cancers 5 were Gleason score 3 + 4 and 1 was score 4 + 3. No Gleason score ≥ 8 cancers were detected. The distribution of PI-RADS and Gleason scores are shown in . Of the 6 Gleason score 7 cancers, 4 were located in the apical and 1 in the apical/anterior part of the prostate ().
Figure 1. Magnetic resonance images from the six men whose cancer was upgraded on targeted biopsy. (A) PI-RADS 4 lesion peripheral zone, dorsolateral, left, apex, Gleason 3 + 4. (B) PI-RADS 3 lesion, peripheral zone, lateral, left, mid, Gleason 3 + 4. (C) PI-RADS 3 lesion, peripheral zone, dorsolateral, right, apex, Gleason 3 + 4. (D) PI-RADS 4 lesion, transition zone, anterolateral, left, apex, Gleason 3 + 4. (E) PI-RADS 5 lesion, peripheral zone, dorsal/dorsolateral, right, mid/apex, Gleason 4 + 3. (F) PI-RADS 4 lesion, peripheral zone, anterior, right, apex, Gleason 3 + 4.
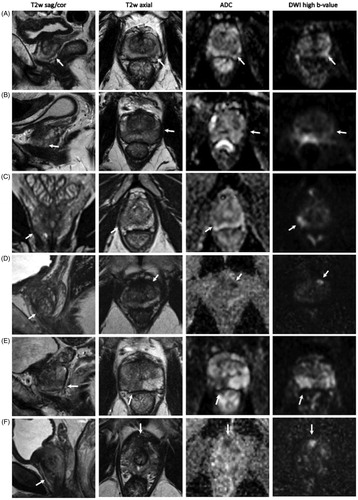
Table 2. The highest PI-RADS score and the cancers detected in the 25 men in the study who had a suspicious MRI.
The targeted biopsies detected a Gleason score 7 cancer in 3 of the 5 men with PSA density ≥ 0.15 ng/ml/cm3, but only in 3 of the 40 men with PSA density < 0.15 ng/ml/cm3 (p = 0.04).
All six men whose cancer was upgraded to Gleason score 7 (ISUP 2 and ISUP 3) were counseled about the pros and cons of continued surveillance versus active treatment. Four of them chose to have a robotic radical prostatectomy, one chose to have external beam radiotherapy and one continued on active surveillance because of significant comorbidity. The results after surgery are shown in .
Table 3. Biopsy results in upgraded patients and choice of treatment with postoperative Gleason score when applicable.
Discussion
This prospective trial to evaluate the benefit of a first MRI and targeted biopsies in men who have been on AS for several years for a low-grade prostate cancer, without signs of progression during surveillance, showed upgrading to a Gleason score 7 cancer in almost one-sixth of the men. Upgrading was much more common in men with a PSA density above 0.15 ng/ml/cm3. Nearly, all Gleason score 7 cancers were located in the apical or anterior parts of the prostate. It is remarkable that despite several years of AS and a total number of 1640 of systematic biopsy cores during surveillance, MRI and only 101 targeted biopsy cores revealed several cancers of a type that in 83% (5/6) of the patients were considered serious enough to lead to a recommendation of curative treatment.
MRI was already in 2014 recommended in the NICE guidelines for the assessment of men who start on AS [Citation8]. MRI is now also recommended in the European and the Swedish guidelines [Citation2,Citation9]. However, the recently reported randomized trial Active Surveillance Magnetic Resonance Imaging Study (ASIST) showed no difference in upgrading to Gleason score ≥7 between men who had a standard confirmatory transrectal systematic biopsy and men who had an MRI with targeted biopsies [Citation10]. The authors acknowledged that the negative results probably were influenced by that the involved clinicians were relatively inexperienced with image fusion technology and that usually only one, sometimes two, targeted biopsies were taken from each lesion. Previous studies have shown benefits of using MRI and fusion-guided biopsies compared with a standard 10–12 core biopsy for the detection of Gleason score ≥7 cancer [Citation11,Citation12] and reduced detection of low-grade cancer [Citation13]. A meta-analysis showed that a positive MRI with targeted biopsies was more likely to identify clinically significant disease [Citation14]. A recently published two-year follow-up of the ASIST trial showed, in contrast to the first report, that MRI before the confirmatory biopsy resulted in 50% fewer active surveillance failures and less progression to higher-grade cancer. The authors’ conclusion was that these results confirm the value of MRI in men on surveillance [Citation15].
The question is whether the Gleason score 7 cancers found with MRI and targeted biopsies in our study were examples of true disease progression or if they were present and missed at the time of previous systematic biopsies. The fact that almost all these high-grade cancers were located at the apex of prostate and one-third anteriorly suggests the latter, as MRI and targeted biopsies have a particular benefit over systematic biopsies to identify cancers in these locations [Citation16,Citation17].
Our findings also support previous reports of the importance of PSA density, particularly in combination with MRI [Citation18–20].
Strengths of our study include the prospective design of the SAMS trial and that all eligible trial participants at two centers were included, thus minimizing selection bias. A limitation is the low number of patients, which makes the point estimates of proportions uncertain.
In conclusion, even after several years of active surveillance for low-grade prostate cancer, with many sets of systematic biopsies, a first MRI and targeted biopsies lead to the detection of Gleason score 7 (ISUP 2 and ISUP 3) cancer in a significant proportion of men without rising PSA values, particularly among those with a high PSA density. Upgrading leading to treatment change was seen in 11% of the men. We find these results important, but they need to be interpreted with caution because of the scarcity of available patients.
Acknowledgements
The authors are grateful for the work done by the SAMS research team. To the steering committee: Erik Holmberg, Regional Cancer Centre, Västra Götaland, Gothenburg; Ove Andrén, Örebro University Hospital; Eva Johansson, Academic Hospital, Uppsala; Andreas Josefsson and Maria Nyberg, Sahlgrenska University Hospital, Gothenburg; Jonas Sandberg, Norrland University Hospital, Umeå; Pär Stattin, Uppsala University; David Robinsson, Jönköping County Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–277.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629.
- Bokhorst LP, Valdagni R, Rannikko A, et al.; PRIAS study group. A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70(6):954–960.
- Duffield AS, Lee TK, Miyamoto H, et al. Radical prostatectomy findings in patients in whom active surveillance of prostate cancer fails. J Urol. 2009;182(5):2274–2278.
- Taira AV, Merrick GS, Bennett A, et al. Transperineal template-guided mapping biopsy as a staging procedure to select patients best suited for active surveillance. Am J Clin Oncol. 2013;36(2):116–120.
- Bratt O, Carlsson S, Holmberg E, et al. The Study of Active Monitoring in Sweden (SAMS): a randomized study comparing two different follow-up schedules for active surveillance of low-risk prostate cancer. Scand J Urol. 2013;47(5):347–355.
- Epstein JI, Egevad L, Amin MB, Grading Committee, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244–252.
- Cancer NCCf. NICE guidelines prostate cancer 2014. Available from: https://www.nice.org.uk/guidance/cg175/evidence/full-guideline-191710765
- Prostatacancer – Nationellt Vårdprogram 2019. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/prostatacancer/vardprogram/nationellt-vardprogram-prostatacancer.pdf
- Klotz L, Loblaw A, Sugar L, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol. 2019;75(2):300–309.
- Puech P, Rouviere O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy-prospective multicenter study. Radiology. 2013;268(2):461–469.
- Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015;33(1):17 e1–e7.
- Grönberg H, Eklund M, Picker W, et al. Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. Eur Urol. 2018;74(6):722–728.
- Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67(4):627–636.
- Klotz L, Pond G, Loblaw A, et.al. Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2-year postbiopsy follow-up. Eur Urol. 2020;77(3):311–317.
- Lawrentschuk N, Haider MA, Daljeet N, et al. ‘Prostatic evasive anterior tumours’: the role of magnetic resonance imaging. BJU Int. 2010;105(9):1231–1236.
- Nix JW, Turkbey B, Hoang A, et al. Very distal apical prostate tumours: identification on multiparametric MRI at 3 Tesla. BJU Int. 2012;110(11 Pt B):E694–700.
- Hansen NL, Barrett T, Koo B, et al. The influence of prostate-specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7-10 prostate cancer in a repeat biopsy setting. BJU Int. 2017;119(5):724–730.
- Schoots IG, Osses DF, Drost FH, et al. Reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease. Transl Androl Urol. 2018;7(1):132–144.
- Bhat NR, Vetter JM, Andriole GL, et al. Magnetic resonance imaging-defined prostate-specific antigen density significantly improves the risk prediction for clinically significant prostate cancer on. Biopsy Urol. 2019;126:152–157.
