Abstract
Aim
Evaluation of treatment and survival of pT1 stage (T1) bladder cancer (BC) patients diagnosed with transitional cell carcinoma of the urinary bladder in Norway.
Material and Methods
According to the Cancer Registry of Norway, 1,108 patients were diagnosed with T1 BC between 2008-2012. Information on surgical and medical procedures was provided by the Norwegian Patients Registry. Regression and survival models were applied to characterize patients receiving bacillus Calmette-Guerin (BCG) and radical cystectomy (RC) as early and delayed treatment and to estimate overall and cause specific survival rates (OS; CSS). Adjustments for sex, age, WHO grade and concomitant cis were made.
Results
In total, 449 (41%) patients received BCG treatment, 162 (15%) as early treatment. RC represented the early treatment in 96 (9%) patients and the delayed treatment in 84 (8%). Overall, 850 (77%) patients received neither BCG nor RC as early treatment, of whom 287 (26%) were treated with BCG and 66 (6%) with RC during follow-up. Patients <75 years and patients with high grade tumors or concomitant cis were more likely to receive BCG and RC as early treatment. 5-year survival rates for all T1 BC patients were 84% (CSS) and 65% (OS). Delayed RC was associated with the lowest 5-year CSS (70%). After adjustment, gender did not impact treatment choice and CSS.
Conclusions
The use of BCG as early treatment indicates low adherence to existing guidelines. Delayed RC was associated with low survival rates. An increased focus on the management of T1 patients is needed in Norway.
1. Introduction
Worldwide, 550,000 new bladder cancer (BC) cases were diagnosed in 2018, making BC the sixth most common cancer type for men and the 17th for women [Citation1]. In Norway 1,516 new BC cases were diagnosed in 2018 [Citation2], of which 25% in women. Known risk factors include age, smoking, arsenics in drinking water, radiation exposure and occupational exposure to carcinogens [Citation3].
BC tumor stage T1 is infiltrating lamina propria and comprises 15-20% of all BC tumors [Citation4]. The cancer specific survival (CSS) is reported to be 87% in a metaanalysis [Citation5]. T1 BC represents a tumor stage with several challenges and controversies. The major concerns in the management are the risk of understaging the tumor at the primary diagnostic transurethral resection of the tumor (TURB) and the risk of tumor progression into muscle invasive BC (MIBC) [Citation6]. The 5-year progression rate in T1 tumors is about 20% [Citation5,Citation7], and the risk of understaging the tumor at diagnose is 8%, although with high variation across studies (0–32%) [Citation8]. The routine use of repeated TURB (reTURB) within the first 4–6 weeks after diagnosis has therefore been recommended in the European association of Urology (EAU) guidelines since 2008 [Citation9].
For most T1 patient conservative treatment after the initial TURB and reTURB represents the early treatment, which according to EAU guidelines comprises repeated intravesical Bacillus Calmette–Guérin (BCG) instillations and cystoscopy surveillance. BCG treatment reduces both progression rates and tumor relapses [Citation4,Citation10]. However, conservative treatment is not the optimal early management for all T1 patients, and the challenge is to identify the sub-group of T1 patients who should be offered radical cystectomy (RC) as early treatment in order to prevent progression and thereby hopefully obtain favorable survival [Citation6]. To help identifying the T1 patients with high risk of progression EAU guidelines give a set of risk factors highly associated to progression. In the 2008 guideline risk factors included were high grade (HG) tumors, large and multiple tumors and concomitant carcinoma in situ (cis) [Citation4,Citation9]. Additional parameters such as the age and comorbidities of the patients along with morbidity and mortality associated with RC are considered when clinicians make treatment decisions. Therefore, the management of T1 patients is complicated and requires close collaboration with several medical specialists involved.
To our knowledge, no population-based studies are available addressing treatment and survival outcome in T1 patients diagnosed in Norway. Thus, with focus on early treatment we describe management and survival in a population-based cohort of T1 BC patients in order to identify diagnostic or therapeutic tasks of future improvement.
2. Material and methods
2.1. Material
2.1.2. Data sources
The Cancer Registry of Norway (CRN) has since 1953 registered new cancer diagnoses in Norway. The registry receives information from several independent sources (clinicians, pathology laboratories, radiation machines, Norwegian Patient Registry and the Cause of Death Registry), thus ensuring high completeness of data [Citation11]. Patients were identified through the personal identification number assigned to all newborns and residents in Norway since 1960. Cases were selected based on morphological snomed CT codes for transitional cell carcinoma of the urinary bladder. All histological reports for BC patients diagnosed between 2008 and 2012 and subsequent reports until the 31st of December 2016, registered in the CRN, were quality insured by the research team. Type of surgery, stage, WHO grade ([Citation12]), concomitant cis and the presence of muscle in the tissue specimen were determined.
The Norwegian Patient Registry includes administrative data on all patients from publicly financed hospitals as well as private hospitals and specialists, as a supplement to services at the public hospitals. For every study patient, we obtained dates for medical and surgical procedures related to the BC diagnosis such as TURB, reTURB, BCG treatment and RC. ReTURB was defined as a TURB within 12 weeks after the initial T1 diagnosis. As the coverage for the ATC code for BCG treatment was low, we defined BCG treatment either by the ATC code (L03AX03) or as at least three subsequent intravesical treatments with not more than 15 days in between two subsequent intravesical treatments. In order to differentiate BCG from intravesical treatment with Mitomycin, we excluded intravesical treatment given the day of TURB. First-line BCG treatment was defined as BCG treatment within 8 weeks after T1 diagnosis and first-line RC as RC within 6 months after the T1 diagnosis.
2.1.3. Study population
We identified 1,506 patients with an initial T1 urothelial carcinoma of the urinary bladder diagnosed by TURB between 2008 and 2012 in the CRN. We excluded 144 patients with another cancer diagnosis 5 years prior to the BC diagnosis and 254 patients because of upstaging of the T1 tumor within 4 months. Upstaging was defined as MIBC diagnosis by reTURB or metastatic disease within 4 months after the initial T1 diagnosis. In total, the study population comprised 1,108 T1 BC patients which were followed for treatment and outcome until death, migration or end of follow up on the 30th of June 2017, whichever came first. The total follow-up time was 15,260 person-years with a median follow-up time of 5.8 years. The last update of cause of death was on December 31st of 2016.
2.2. Statistics
Descriptive statistics and survival models were used to evaluate management and survival of the study population. Risk factors potentially influencing the early treatment were evaluated by (logistic) regression models, CSS and overall survival (OS) by applying flexible parametric models [Citation13,Citation14]. When evaluating risk factors for early treatment choice, we included sex, age, WHO grade, concomitant cis, reTURB, presence of muscle in the diagnostic histological and whether the patients had another cancer diagnosis more than 5 year before BC diagnosis into the multivariate model. Age, sex and all relevant risk factors (p < 0.20 in the risk factor analysis ) was adjusted for in the survival analyses. The baseline hazard was modeled using 4 degrees of freedom (df) for the spline variables using the Stata command stpm2 [Citation15]. After fitting the model, excess mortality rates could be estimated for any covariate pattern. The quantities reported are the hazard ratios (HRs) including 95% confidence intervals (CIs) and p-values.
Table 1. Characteristics of T1 BC patients diagnosed from 2008-2012 in Norway.
3. Results
3.1. Patient, tumour and treatment characteristics
Patient characteristics of the 1,108 T1 BC patients are provided in . Median age at diagnosis was 75 years with women being 2 years older (76 vs. 74 years). More men than women were diagnosed with HG tumors, concomitant cis, and with higher rate of muscle tissue in the specimen. In total 180 patients (16%) had RC of which 96 (9%) as early treatment. 42% of the patients received BCG but only 15% as early treatment. Cis was reported in 13% of the histologies. In total 52% of the patients had a reTURB. During the study follow-up, 175 (16%) patients died of BC and 299 (27%) of other causes.
illustrates the numbers of patients treated with early conservative treatment (with and without BCG) or RC in addition to the numbers of patients receiving delayed RC and BCG treatment . The figure is also reflecting the timeframe of subsequent delayed RC or BCG treatments with majority of treatments given the first year after the early treatment. The tumor stage at RC revealed T0 for 14, Tis for 13, Ta for 5, T1 for 7 and MIBC for 31 patients. In , we present patient characteristics which are associated with early treatment. Older patients were significantly less likely to receive RC or BCG treatment (when compared to conservative treatment without BCG). The presence of a HG tumor and concomitant cis increased the probability of both RC and BCG treatment.
Figure 1. Early and delayed treatment for T1 BC patients. Treatments considered are conservative treatments with and without BCG and RC. The histograms show the distribution of time since diagnosis of the respective delayed treatments.
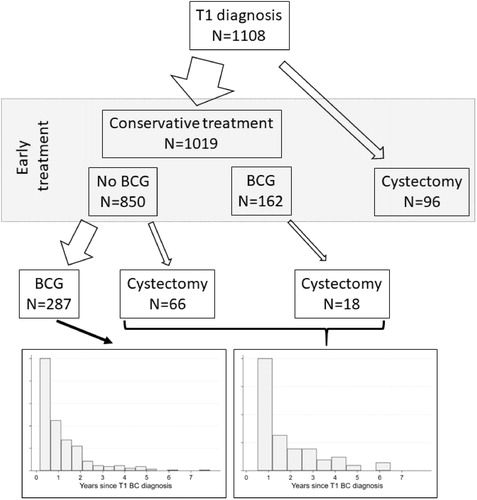
Table 2. Patient characteristics for T1 BC patients receiving conservative treatment or cystectomy as early treatment. P-values indicate the effect of the respective patient characteristics on early BCG and RC treatment choice when compared to conservative treatment without BCG in a multivariable model.
3.2. Survival analyses
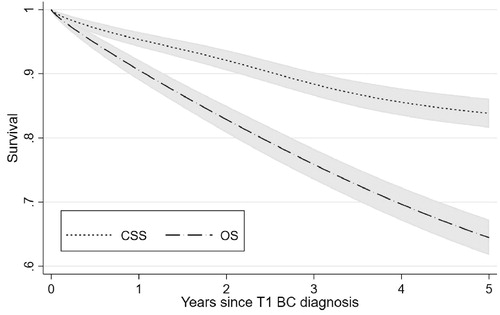
CSS and OS estimates including confidence intervals are shown in . The 1-, 2- and 5-years survival estimates for CSS were 95%, 92% and 84% respectively and the corresponding estimates for OS were 91%, 83% and 64%. Results from the survival analyses are presented in . After adjustment for clinical parameters, BCG treatment was significantly associated with better OS (p = 5.3·10−3) and to less extent (p = 0.094) with better CSS when compared to conservative treatment without BCG. Early RC was not associated with CSS (p = 0.47) or OS (p = 0.16). HG tumors (vs. LG tumors) at diagnosis were directly related to worse outcome (CSS: p = 0.034; OS: p = 3.6·10−5). Concomitant cis did not impact survival. Women had a significantly better OS than men.
Table 3. Results from the cause-specific and overall survival analysis. A hazard ratio HR > 1 indicates a higher mortality risk compared to the reference group, HR < 1 a lower risk respectively. We also provide confidence intervals (CI) and p-values.
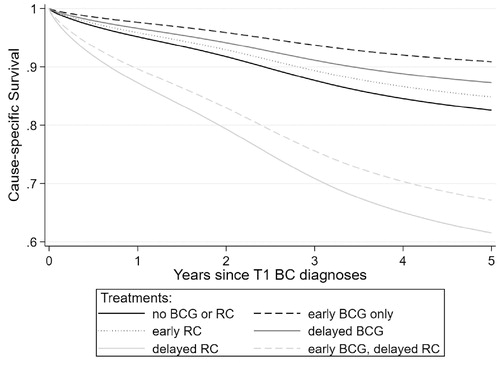
shows CSS dependent on combined information on early and delayed treatment. Patients receiving conservative early treatment and a delayed RC had the lowest 5-year CSS: 73% (CI: 56–94%), for those treated early conservative with BCG and 61% (CI: 51–74%) for those early conservative without BCG. The best 5-year survival rates were observed for the group receiving only early BCG treatment (91%; CI: 86–96%) and the group with conservative early treatment without BCG and delayed BCG(87%; CI: 83–91%). 5-year CSS estimates for early RC were 85% (CI: 76–94%) and for the group both with neither early nor delayed (BCG or RC) treatment: 83% (CI: 79–86%).
4. Discussion
To our knowledge, this is the first Norwegian retrospective register-based study examining treatment and survival of patients with T1 transitional cell carcinoma of the urinary bladder. All patients were diagnosed between 2008 and 2012 at a median age of 75 years. The main findings were a relatively low rate of early BCG treatments (15%) and an overall high rate of RC (16%). 5-year CSS and OS rates were 84% (CI: 82–86%) and 64% (CI: 62–67%). The lowest CSS rates were found in patients undergoing RC more than 6 month after diagnosis. Patients older than 75 years were less likely to receive both BCG and RC as early treatment.
T1 BC is a heterogenic tumor demanding high quality in diagnostic work-up and therapeutic decisions to secure the correct diagnosis and optimal management [Citation16]. The quality of the diagnostic procedures is generally assessed by the presence of muscle tissue in the TURB specimen and the rate of patients receiving reTURB. We report a reTURB rate of 52%, and 87% of the patients had muscle in the histological specimen, obtained by the primary or at the reTURB histology, which means that 13% had their T1 diagnose without any muscle in the tissue.
BCG is the internationally recommended conservative treatment for T1 BC patients [Citation4] and is the only bladder instillation treatment shown to reduce progression rates of T1 tumors, but with modest effect, reducing progression rate from 13 to 9%, a risk reduction of 27% [Citation10]. Although controversies regarding impact of progression [Citation17,Citation18] and survival [Citation4] BCG has remained essential in T1BC treatment for decades [Citation19]. Our total rate of T1 patients having BCG either as early or as delayed treatment is 41%. However, only 15% received BCG as early treatment (within 8 weeks after final diagnosis), as recommended in the EAU guidelines. The age distribution presented in a Swedish T1 nation-wide population-based study was similar to ours, but the BCG rate was higher, about 50% [Citation20]. The reason for the overall low adherence to guidelines in our study could be caused by high age and comorbidities of the patients as well as the urologist’s disbelief with respect to effectiveness of BCG treatment and fear of severe side effects.
In total, 180 (16%) of the study population had RC. The SEER (The Surveillance, Epidemiology, and End Results) database with 8,476 T1 patients diagnosed from 2004 to 2007, reported a RC rate of 4.7% within the first year after diagnosis [Citation21]. A Swedish cohort reported 6.8% cystectomies for T1 WHO Grade (G) 2/3 cases, 11% when including only T1G3 [Citation22]. The latest number of RC in the Swedish National Registry for Urothelial Bladder cancer (SNRUBC) was 12% for T1G2/3, 14% for only T1G3, in 2017 [Citation23]. Compared to the referred studies our percentage of cystectomies is higher. We do not expect the low number of LG cases (10%) to impact the overall results as most RC cases are HG tumors.
T1 patients at a particularly high risk of tumor progression into MIBC should, according to EAU guidelines, be assessed for and offered RC when fit for it. Correct timing of the RC is crucial for optimal treatment results. In our study, 31 (44%) out of 70 patients with delayed RC and available histology had a MIBC at RC. Delayed RC at tumor relapse is reported to decrease 10 years CSS from 78% to 51% compared to early RC [Citation24]. Delayed RC after progression to MIBC is followed by lower survival rates than RC for primary MIBC [Citation25,Citation26]. In agreement with these published survival rates, we report the lowest CSS rate for patients receiving delayed among our treatment groups. Delayed RC in our study comprised about half of the total number of RCs. It could be questioned whether both the early and the follow-up management of these patients have been optimal. Many of these patients have probably undergone RC too late. But to determine the most appropriate date of RC is difficult, since both urologists and the patients want to avoid overtreatment with the possible morbidities as well as mortality associated to RC. At the same time, RC may for some patients be the only chance for cure [Citation6]. Survival of T1 study patients with early RC was close to that of the two groups with the best CSS; i.e. patients with early BCG only and those with delayed BCG. Whether any of these patients with RC as early treatment were over-treated is impossible to evaluate from this study.
Survival estimates for T1 patients vary considerably in the literature, reflecting differences in study populations and methods from modeling survival. In the Norwegian population, 16% of the T1 patients died of BC during the study period with a median follow up time of 5.8 years. Sjostrom et al reported BC-related death of 18% in a T1 cohort, but patients with primary RC were excluded [Citation20]. Another Swedish study reported 5-year relative survival of 76% for T1 patients in the period 2007–2011 [Citation27]. In our study, we observed a 5-year relative survival of 81% (CI: 77–84%) (results not shown).
Both a tumor with HG (compared to LG) and concomitant cis led to significantly more early BCG and RC treatment and were thus, as expected, identified as risk factors considered in treatment decisions for T1 cases [Citation4]. We could not confirm any impact of concomitant cis on survival. Our rate of concomitant cis is rather low (13%) when compared to about 25% in other reports [Citation28,Citation29].
It has been reported, that women are at risk to undergo sub-optimal treatment of BC in general more often than men, and that they have a less favourable prognosis [Citation30]. Another study also showed inferior treatment of women with T1 BC diagnosis as they had less BCG treatment and a lower relative survival when compared to men [Citation20]. In agreement with these observations, we found that a higher proportion of men received both RC and BCG treatment. This could be caused by a higher rate of HG tumors and more concomitant cis in men. Less muscle in the TURB specimens in women (78 vs. 71%) could also potentially lead to more understaging in women leading to lower rates of BCG and RC treatment. However, in contrast to other studies, we did not find any gender differences related to treatment choice or CSS, after adjustment for clinical parameters.
A limitation in addressing management of T1 BC in a retrospective manner is the lack of essential information about comorbidity, imaging information and important risk factors for progression such as size and number of tumors, which influence both treatment choice and survival. Another limitation is the lack of a systematic depth categorization of tumor infiltration in the histological report. Information given by the histological report were tumor stage, grade, concomitant cis and presence of muscle in the specimen. The findings of this study must be interpreted on the background, that the first Norwegian national guidelines for BC management were published in 2018 with international guidelines available, like the EAU guidelines since 2000.
In conclusion, we report a low adherence to guideline recommendations regarding early BCG treatment for T1BC. The rate of RC was higher than comparable Swedish numbers. About half of the RC patients had RC as early treatment with good survival results, while patients with delayed RC had the worst CSS suggesting inferior early and/or follow-up management. Our results suggest an increased focus on both early and follow-up management of T1 patients.
Author contributions
BKA conceived, coordinated and designed the study, performed the statistical analysis and drafted the manuscript. RB and TAM participated in the statistical analysis and interpretation of the analysis. AB was responsible for the clinical assessment of the patients included and contributed in the interpretation of the results, background knowledge on bladder cancer and writing the manuscript. All authors participated in writing of the manuscript and revised it critically. All authors have read and approved the final version of the manuscript.
| Abbreviations | ||
| BC | = | Bladder Cancer |
| CI | = | Confidence Interval |
| CRN | = | Cancer Registry of Norway |
| MIBC | = | Muscle-Invasive Bladder Cancer |
| BCG | = | Bacille Calmette Guérin |
| cis | = | Carcinoma in Situ |
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Cancer Registry of Norway. Cancer in Norway 2018 - Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2019.
- Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784–795.
- Babjuk M, Burger M, Comperat EM, et al. European association of urology guidelines on non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol. 2019;76(5):639–657.
- Martin-Doyle W, Leow JJ, Orsola A, et al. Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients. J Cin Oncol. 2015;33(6):643–650.
- Klaassen Z, Kamat AM, Kassouf W, et al. Treatment strategy for newly diagnosed T1 high-grade bladder urothelial carcinoma: new insights and updated recommendations. Eur Urol. 2018;74(5):597–608.
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466. discussion 475–7.
- Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2018;73(6):925–933.
- Babjuk M, Oosterlinck W, Sylvester R, et al.;European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54(2):303–314.
- Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168(5):1964–1970.
- Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231.
- Montironi R, Lopez-Beltran A, Scarpelli M, et al. 2004 World Health Organization Classification of the Noninvasive Urothelial Neoplasms: Inherent Problems and Clinical Reflections. European Urology Supplements. 2009;8(5):453–457.
- Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23(1):51–64.
- Nelson CP, Lambert PC, Squire IB, et al. Flexible parametric models for relative survival, with application in coronary heart disease. Statist Med. 2007;26(30):5486–5498.
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. The Stata J. 2009;9(2):265–290.
- Azzouz H, Cauberg EC, De Reijke TM. Controversies in the management of T1 urothelial bladder cancer. Minerva Urol Nefrol. 2011;63(4):309–315.
- Schmidt S, Kunath F, Coles B, et al. Intravesical bacillus calmette-guérin versus mitomycin C for Ta and T1 bladder cancer . Cochrane Database Syst Rev. 2020;1:CD011935.
- Kamat AM, Porten S. Myths and mysteries surrounding bacillus Calmette-Guerin therapy for bladder cancer. Eur Urol. 2014;65(2):267–269.
- Alhunaidi O, Zlotta AR. The use of intravesical BCG in urothelial carcinoma of the bladder. Ecancermedicalscience. 2019;13:905
- Sjostrom C, Thorstenson A, Strock V, et al. Treatment according to guidelines may bridge the gender gap in outcome for patients with stage T1 urinary bladder cancer. Scand J Urol. 2018;52(3):186–193.
- Canter D, Egleston B, Wong YN, et al. Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: a SEER database analysis. Urol Oncol. 2013;31(6):866–870.
- Thorstenson A, Hagberg O, Ljungberg B, et al. Gender-related differences in urothelial carcinoma of the bladder: a population-based study from the Swedish National Registry of Urinary Bladder Cancer. Scand J Urol. 2016;50(4):292–297.
- Swedish National Registry of Urinary Bladder Cancer. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/urinvagar/urinblase–och-urinrorscancer/rapporter/urinblasa_arsrapport_2018_final.pdf.
- Denzinger S, Fritsche HM, Otto W, et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach?. Eur. Urol. 2008;53(1):146–152.
- Schrier BP, Hollander MP, van Rhijn BW, et al. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004;45(3):292–296.
- Moschini M, Sharma V, Dell'oglio P, et al. Comparing long-term outcomes of primary and progressive carcinoma invading bladder muscle after radical cystectomy. BJU Int. 2016;117(4):604–610.
- Jahnson S, Hosseini Aliabad A, Holmang S, et al. Swedish National Registry of Urinary Bladder Cancer: No difference in relative survival over time despite more aggressive treatment. Scand J Urol. 2016;50(1):14–20.
- Gontero P, Sylvester R, Pisano F, et al. The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette-Guérin. BJU Int. 2016;118(1):44–52.
- Palou J, Pisano F, Sylvester R, et al. Recurrence, progression and cancer-specific mortality according to stage at re-TUR in T1G3 bladder cancer patients treated with BCG: not as bad as previously thought. World J Urol. 2018;36(10):1621–1627.
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69(2):300–310.