Abstract
Purpose
Locally advanced prostate cancer can cause bladder outlet obstruction, gross hematuria and frequent hospitalization. While these complications are commonly treated by palliative transurethral resection of the prostate, the improvement is often insufficient. The purpose of this study was to evaluate the safety and feasibility of MRI-guided transurethral ultrasound ablation as an alternative palliative treatment option (pTULSA) for men suffering from symptomatic locally advanced prostate cancer.
Methods
This prospective, phase one study included 10 men in need of palliative surgical intervention due to urinary retention and gross hematuria caused by locally advanced prostate cancer. Patients were followed for 1 year at 3-month intervals. Time without catheter, time without hematuria, reduction in hospitalization time, and adverse events were measured.
Results
Ten patients with locally advanced prostate cancer were enrolled, all having continuous catheterization due to urinary retention and nine had gross hematuria before treatment. At 1 week post-pTULSA five patients were catheter-free. At last follow-up catheter-free and gross hematuria-free rates were 70% and 100%, respectively. Average hospitalization time from local complications reduced from 7.3 to 1.4 days in the 6 months before and after pTULSA. No > Grade 2 treatment related adverse events were reported, with all five being urinary tract infections.
Conclusions
pTULSA appears safe and feasible for palliative ablation of locally advanced prostate cancer. The therapy seems to accomplish long-term hematuria control, can relieve bladder outlet obstruction in selected patients, and seems to reduce the burden of hospitalization due to local complications. Trial Registration Number: NCT03350529
Introduction
Many men develop locally advanced prostate cancer (LAPCa), either from a late diagnosis or as a recurrent disease after primary therapy. These patients may suffer from bladder outlet obstruction (BOO), urinary tract infections (UTI) and gross hematuria. The objective of palliative LAPCa management involves local disease control, relieving local symptoms, reducing hospitalization and improving quality-of-life (QoL) [Citation1].
To relieve local symptoms the current gold standard is palliative transurethral resection of the prostate (pTURP). However, pTURP carries notable surgical and anesthetic risks which increase with age [Citation1–5] and may exclude those who cannot discontinue anticoagulants. Other interventions include androgen deprivation therapy (ADT), radiation therapy (RT) [Citation6,Citation7] and palliative radical surgery [Citation8], each with their own set of challenges. There is currently an unmet need for minimally invasive palliative treatments for men with local symptoms arising from LAPCa, particularly those who are at an advanced age, have increased burden of comorbidities and have low performance status [Citation9,Citation10]. MRI-guided transurethral ultrasound ablation (TULSA) is one such possibility. Combining MRI treatment planning and real-time temperature monitoring, TULSA generates conformal ultrasound ablation in the prostate via a transurethral ultrasound catheter. Previous TULSA studies have demonstrated oncological efficacy among men with localized PCa with a favorable safety profile and low impact on QoL [Citation11–13].
The primary objectives of this study were to evaluate the safety and feasibility of palliative TULSA (pTULSA) as an alternative local therapy for treating gross hematuria and/or urinary retention in patients with LAPCa.
Materials and methods
Study design
This was an investigator-initiated, prospective, non-randomized, single-arm, and single-center Phase-I study evaluating the early stage safety and feasibility of TULSA as a palliative intervention for LAPCa (NCT03350529). For this reason, only 10 patients were enrolled, and no comparative arm was used. The study protocol was approved by the ethics committee of the Hospital District of Southwest Finland and written informed consent was obtained from all participants. The trial was performed in accordance with the principles of the Declaration of Helsinki.
Patient eligibility and selection
Study patients were identified for this study by the Department of Urology at the Turku University Hospital. The patient selection included those patients presenting with either primary or radiorecurrent LAPCa (with or without metastasis) that were referred to the urological outpatient clinic or admitted to the hospital due to local complications. A life expectancy greater than 3 months was required. Local complications that required palliative treatment included ongoing/recurrent gross hematuria and/or urinary retention requiring continuous catheterization, which were not resolving by conservative or medical treatment.
Description of the intervention
Treatment was delivered using TULSA (TULSA-PRO, Profound Medical Inc., Mississauga, Canada). A detailed description of the intervention is described in our previous study assessing TULSA for lesion-targeted ablation of localized PCa [Citation11]. Due to the heterogeneity of the study participants, the treatment approach was dependent on the individual disease characteristics. If visible, the ablation was targeted to the dominant tumor section compressing and/or invading the prostatic urethra, otherwise the objective was to debulk the prostate. As more experience was obtained, any tissue obstructing the bladder neck was also targeted, regardless of if a tumor was present.
Follow-up and assessment
Follow-up visits occurred at 1 week, 3, 6, 9 and 12 months. Safety was assessed by recording adverse events at every follow-up visit using Clavien Dindo Classification for Surgical Complications. Preparations were made at study onset to systematically collect QoL and functional questionnaires, as well as uroflowmetry measurements at each follow-up visit. However, due to the poor health of the study population, many items were missing and therefore omitted from the overall feasibility assessment. Instead feasibility was assessed by examining if pTULSA was able to treat gross hematuria and/or BOO. Time without catheter, time without hematuria and any reduction in hospitalization time were all assessed post-pTULSA. Catheter removal and an initial voiding trial was performed 1 week after pTULSA. At least one cystoscopy was performed to assess the treatment effect and patency of the urethra.
Results
Patient characteristics
Ten patients were treated with pTULSA between November 2017 and June 2019. The characteristics of the study population are presented in . The median (range) time interval between the initial PCa diagnosis and pTULSA was 30 months (4–194). All patients were Caucasians. Half of the patients presented with clinical T4 tumors, while the other half had T3 tumors. Four patients with T4 tumors had direct invasion of the tumor in the bladder neck and/or posterior bladder wall. Individual pre-treatment prostate MRI images are presented in Supplementary Figure S1.
Table 1. Patient characteristics at baseline.
Prior to pTULSA, six patients had undergone external beam RT combined with 2–3-year ADT, while the other four patients were treated only with ADT. At enrollment eight patients had metastatic disease, five of which had castration-resistant PCa. The median (range) age, CCI-score, ECOG PS, baseline PSA and prostate volume were 76.5 years (60–81), 10.5 (5–15), 2 (1–3), 18.5 ng/mL (0.23–140) and 35 cc (12–213), respectively.
Local symptoms and complications prior to pTULSA
Prior to pTULSA all patients had continuous indwelling catheterization due to urinary retention. Nine patients also had a history of recurrent and/or ongoing gross hematuria. Three patients had a pTURP performed 6 months prior to receiving pTULSA, all of which were unsuccessful. The baseline median (range) post-void residual volume (PVR) from eight patients was 393 mL (300–1000). Two patients with large retention were unable to void spontaneously so PVR was not possible. Two patients had hydro nephrosis.
Study intervention
pTULSA was technically feasible in every study patient with a mean ablation time of 37 min (range = 16–58) and targeted ablation volume of 30.6 cc (range = 12–84). No difficulties with device instrumentation were encountered. Two patients had a suprapubic catheter (SPC) during pTULSA, while the others did not have urinary drainage during the procedure, receiving a transurethral catheter afterwards. One patient was discharged on the treatment day, and another patient on the second postoperative day. The rest (n = 8) were discharged on the first postoperative day. The average (range) hospitalization time was 28.6 h (12–48).
Safety and treatment related morbidity/toxicity
Two Grade 2 and three Grade 1 adverse events were recorded according to Clavien Dindo Classification of Surgical Complications, all related to UTI. Two patients were hospitalized requiring intravenous administration of antibiotics, while the remaining UTI resolved without hospitalization on oral antibiotics alone. No grade 3 or higher adverse events were recorded, with no rectal injury or fistula. Further, there was no need for blood transfusions and there was no perioperative mortality.
Feasibility of pTULSA
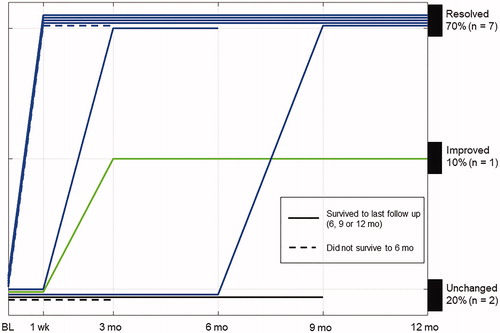
Catheter removal at 1 week post-pTULSA was successful in five patients with a median (range) PVR 113 mL (82–151). Two patients had their SPC removed at 3 and 9 months after sufficient evidence of adequate bladder emptying was displayed. demonstrates the temporal evolution of catheterization for all study patients at baseline and after pTULSA. Three patients underwent pTURP after pTULSA because of persistent BOO. At the last follow-up visit 70% (7/10) of the study patients were catheter-free, five patients after pTULSA alone and two patients after additional pTURP. One of the three patients undergoing post-TULSA pTURP had no improvement on BOO and continued clean intermittent catheterization (CIC). Two patients remained continuously catheterized after pTULSA ().
Table 2. Feasibility of pTULSA.
Due to an initial conservative treatment approach around the bladder neck, three of the first four patients treated had persistent BOO after pTULSA. Once the treatment plan was modified to always target the bladder neck no additional patients underwent a pTURP after pTULSA. Those three patients underwent a pTURP targeted to the bladder neck 3 months after pTULSA, with varying success. The first patient was catheter-free the first 3 months after pTULSA but developed rising PVR and underwent a pTURP, shortly thereafter discontinuing catheterization. The second patient, who had 2.5 l urinary retention at baseline, remained catheterized after pTULSA. He underwent a pTURP and eventually discontinued catheterization at 9 months post-pTULSA. The third patient improved to CIC after pTULSA, but later underwent a pTURP due to retention, which was unsuccessful in relieving the symptoms. No catheterization improvement was recorded for two study participants, one of them refusing catheter removal due to sedentary condition caused by rapidly progressing disease. Individual treatment plans are presented in Supplementary Figure S2.
Gross hematuria ceased in all study patients at 1 week post-pTULSA, continuing without occurrence of gross hematuria (10/10) until the last follow-up visit (). Comparing the 6 months preceding pTULSA to 6 months after the average hospitalization, time (range) due to local complications decreased from 7.3 days (0–20) before pTULSA to 1.4 days (0–7) after pTULSA.
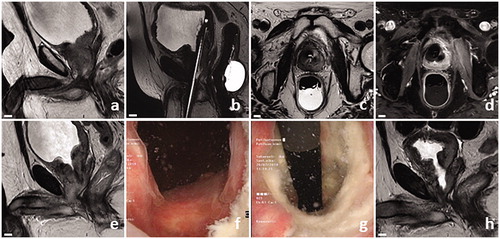
describes a successful pTULSA case. A 65-year-old male with metastatic and castration-resistant LAPCa, suffering from unresolved urinary retention and recurrent gross hematuria, underwent pTULSA. From 1 week until 12 month follow-up the patient survived free of catheter and gross hematuria.
Figure 2. Successful pTULSA case example. Patient presented with radio recurrent castration-resistant LAPCa (rT4N1M1b, GGG5, PSA 19), was continuously catheterized, had a history of recurrent gross hematuria, and the prostatic urothelium line was bleeding from cystoscopy contact. Baseline sagittal MRI image showed obstructive infiltration of PCa to the bladder neck and posterior bladder wall (a). Treatment-day MRI planning images showing the transurethral ultrasound applicator and endorectal cooling device (b and c). 19 cc of tumor around the bladder neck was targeted, with contrast-enhanced MRI revealing immediate effects of ablation (d). Transurethral catheter removal was successful at 1 week, accompanied with a prostate volume increase from 35 to 40 cc on MRI (e). Cystoscopy revealed an open bladder neck at 3 months (f and g). A clear cavity could be seen around the proximal prostatic urethra on MRI, with a corresponding decrease in prostate volume to 12 cc at 12 months (h).
Discussion
This is the first study evaluating TULSA for treatment of local symptoms and complications due to LAPCa in a palliative setting. We are not aware of any other prospective studies that used therapeutic ultrasound to treat gross hematuria and/or urinary retention in men with LAPCa. We were able to ablate bulky tumors, some of which infiltrated into the bladder neck and further into the bladder wall.
Catheter removal was successful for five patients at 1 week post-treatment and 50% of patients were free of catheter at 1 year without any subsequent pTURP intervention. The relief of anatomical obstruction after TULSA is slower compared to TURP because tissue ablated by TULSA gradually disappears by sloughing of necrotic tissue into the urinary tract and eradication of ablated necrotic tissue via the hematogenic route. Recent studies have reported that, despite an initial increase in prostate volume as a result of thermal injury derived inflammation, at 3 weeks the prostate volume starts to decrease [Citation11]. This suggests that anatomical obstruction relief is obtained gradually, which should be considered when defining the duration of post-TULSA catheterization. The earlier than expected benefit observed for these five patients may be due to the alleviation of functional obstruction.
In LAPCa, hematuria can be related to BOO or direct invasion of PCa into the urinary tract, which is difficult to manage and can require repeat hospital admissions. Previous RT may also cause radiation urethritis or cystitis which may result in bleeding, and the ongoing use of anticoagulants compounds this risk. Prostate arterial embolization has been used to treat hematuria in a variety of prostatic diseases with reported clinical successes between 83 and 100% [Citation14]. In one small LAPCa-specific study the authors reported a 67% clinical success rate for the treatment of hematuria [Citation15]. Palliative RT has also been used to treat hematuria in LAPCa, where it was demonstrated that 67% of patients showed clinical resolution of 3 months [Citation6]. In the current study, recurring/ongoing gross hematuria ceased for all nine patients who had this symptom before pTULSA, with the added benefit pTULSA can also potentially alleviate BOO in an outpatient setting with a relatively short hospitalization time.
There are some inherent advantages of TULSA compared to existing surgical interventions to relieve hematuria and BOO caused by LAPCa. During TURP prostatic tissues are resected which may potentially cause tumor spillage and systemic tumor dissemination [Citation16,Citation17]. Alternative surgical treatment options including palliative prostatectomy and cystoprostatectomy with urinary diversion may be offered, however, due to their technical complexity, are only available at highly experienced centers and for patients with good performance status [Citation8,Citation18]. Non-surgical options including ADT or other systemic therapies can achieve notable response in metastasis, but rarely affect the prostate itself [Citation18]. TULSA in contrast is a minimally invasive technology which enables bloodless incision-free ablation of prostatic tissue, with an ablation pattern that is customized to each patient’s specific anatomical and functional needs. The real-time MRI image-guidance aspect of TULSA offers accurate visualization of the underlying pathology, while still allowing the user to monitor safety.
Limitations of this study included small sample size and non-randomized setting. On the other hand, there is overall limited data in the topic of local palliative intervention in PCa and recruitment is challenging. The study of palliative interventions also includes challenges in measuring validated efficacy outcomes in a patient population with rapidly declining health. We therefore focused on time without catheter, time without hematuria, and hospitalization reduction.
pTULSA appears safe and feasible for palliative ablation of LAPCa. Long-term control of hematuria was demonstrated, hospitalization was reduced and in certain cases pTULSA relieved lower urinary tract obstruction. More robust data is needed to confirm the efficacy of pTULSA in LAPCa.
Ethical approval
The study protocol was approved by the ethics committee of the Hospital District of Southwest Finland. The trial was performed in accordance with the principles of the Declaration of Helsinki.
Supplemental Material
Download PDF (395.5 KB)Supplemental Material
Download TIFF Image (668.6 KB)Acknowledgments
We thank all the patients and referring physicians whose participation made this study project possible. We thank the entire staff team in the Departments of Medical Physics, Urology and Diagnostic Radiology at the Turku University Hospital. We want to also thank the staff team of the urological outpatient clinic, especially Sara Karnell and Kaisa Reunanen, at Turku University Hospital for their contribution on the project. Without their help and support the timely completion of this project would not have been possible.
Disclosure statement
Dr. Anttinen reports grants from the Finnish Urological Association, grants from the Finnish Urological Research Foundation, during the conduct of the study; grants from Profound Medical Inc, outside the submitted work. Dr. Taimen reports personal fees from Roche, AstraZeneca and MSD, and non-financial support from MSD, all outside the submitted work. Dr. Boström reports grants from the Cancer Foundation Finland, speaker honorariums from Profound Medical Inc and Janssen-Cilag Company, outside the submitted work. The other authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Funding
References
- Heidenreich A, Porres D, Pfister D. The role of palliative surgery in castration-resistant prostate cancer. Oncol Res Treat. 2015;38(12):670–677.
- Marszalek M, Ponholzer A, Rauchenwald M, et al. Palliative transurethral resection of the prostate: functional outcome and impact on survival. BJU Int. 2007;99(1):56–59.
- Crain DS, Amling CL, Kane CJ. Palliative transurethral prostate resection for bladder outlet obstruction in patients with locally advanced prostate cancer. J Urol. 2004;171(2):668–671.
- Pelletier J, Cyr S, Julien A, et al. Contemporary outcomes of palliative transurethral resection of the prostate in patients with locally advanced prostate cancer. Urol Oncol-Semin Ori. 2018;36(8):363.
- Gnanapragasam VJ, Kumar V, Langton D, et al. Outcome of transurethral prostatectomy for the palliative management of lower urinary tract symptoms in men with prostate cancer. Int J Urol. 2006;13(6):711–715.
- Cameron MG, Kersten C, Vistad I, et al. Palliative pelvic radiotherapy for symptomatic incurable prostate cancer–a prospective multicenter study. Radiother Oncol. 2015;115(3):314–320.
- Din OS, Thanvi N, Ferguson CJ, et al. Palliative prostate radiotherapy for symptomatic advanced prostate cancer. Radiother Oncol. 2009;93(2):192–196.
- Pfister D, Porres D, Epplen R, et al. [Palliative radical (cysto)prostatectomy in locally advanced castration-resistant prostate cancer]. Urologe A. 2011;50(9):1101–1105.
- Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29(2):235–241.
- Hehemann MC, Baldea KG, Quek ML. Prostate cancer in the elderly male: diagnostic and management considerations. Curr Geri Rep. 2017;6(3):133–138.
- Anttinen M, Mäkelä P, Suomi V, et al. Feasibility of MRI-guided transurethral ultrasound for lesion-targeted ablation of prostate cancer. Scan J Urol. 2019;53(5):295–302.
- Klotz L, Penson D, Chin J, et al. LBA20 MRI-guided transurethral ultrasound ablation (TULSA) in patients with localized prostate cancer: preliminary results of tact pivotal study. J Urol. 2018;199:1077–1078.
- Chin JL, Billia M, Relle J, et al. Magnetic resonance imaging–guided transurethral ultrasound ablation of prostate tissue in patients with localized prostate cancer: a prospective phase 1 clinical trial. Eur Urol. 2016;70(3):447–455.
- Pereira K, HAlpern JA, McClure TD, et al. Role of prostate artery embolization in the management of refractory haematuria of prostatic origin. BJU Int. 2016;118(3):359–365.
- Chen J-W, Shin JH, Tsao T-F, et al. Prostatic arterial embolization for control of hematuria in patients with advanced prostate cancer. J Vasc Interv Radiol. 2017;28(2):295–301.
- Moreno JG, O'Hara SM, Long JP, et al. Transrectal ultrasound-guided biopsy causes hematogenous dissemination of prostate cells as determined by RT-PCR. Urology. 1997;49(4):515–520.
- Hanks GE, Leibel S, Kramer S. The dissemination of cancer by transurethral resection of locally advanced prostate cancer. J Urol. 1983;129(2):309–311.
- Haidl F, Heidenreich A. The significance of palliative surgery in castration resistant prostate cancer. Aktuelle Urol. 2018;49(5):405–411.