Abstract
Background
Recently, the CARMENA and SURTIME studies, suggested that upfront cytoreductive nephrectomy (CN) should be abandoned for patients with intermediate and high-risk metastatic renal cell carcinoma (mRCC). However, CN remains an indication in low-risk and when immediate systemic treatment is not required. The aim was to evaluate the long-term overall survival (OS) in patients with primary mRCC, based on the first line treatment.
Methods
There were 1483 patients with primary mRCC in the National Kidney Cancer Registry from 2005 to 2013. Data on primary treatment, TNM stage, RCC type, tumor size, patient age and sex were extracted. Survival time was calculated from time of diagnosis to time of death or until July 2019. Mann-Whitney U and Chi-square tests, the Kaplan-Meyer method and Cox regression analyses were used.
Results
Patients primary treated with CN had a significantly longer OS (p < .001) than patients primary treated with systemic therapy or palliation. In a Cox regression multivariate analysis, the hazard ratio for CN compared with no CN was 1.600, 95%Ci (1.492 − 1.691), p < .001. Also occurrence of lymph node metastases, T-stage, patients age and year of diagnosis, remained as independent predictors of OS.
Conclusion
Patients primary treated with CN survived significantly longer than patients primary treated with systemic therapy or palliation, in all age groups. CN was an important first-line treatment option in mRCC patients.
Introduction
Patients with metastatic renal cell carcinoma (mRCC) generally have a poor prognosis [Citation1]. Surgery is potentially curative if all tumor masses are excised, but only a subset of patients with mRCC can be cured with nephrectomy and metastasectomy, in case of single- or oligo-metastatic resectable disease [Citation1]. Thus, for a majority of patients with metastatic disease at diagnosis, cytoreductive nephrectomy (CN) is palliative and systemic treatments are necessary. The value of immediate CN in the treatment of patients with mRCC has therefore been debated. In a meta-analysis of two randomized clinical trials in patients with primary mRCC, a significant survival benefit of CN followed by interferon alfa compared with interferon alone was observed [Citation2]. Similar survival advantage of CN has been observed in multiple studies with thyrosine kinase inhibitors (TKI) reporting a survival benefit of CN combined with TKIs [Citation3,Citation4]. EAU guidelines, based on these data, still recommended CN in patients with a good performance status (PS), absence of poor risk, but also in patients with solitary or oligometastatic disease (Citation1).
The CARMENA study was designed, as a non-inferiority study, to evaluate whether CN is required in the era of targeted therapy in patients with primary mRCC [Citation5]. The results of the prematurely terminated study due to low accrual, indicated, despite a lack of statistical significance, that upfront systemic treatment was non-inferior to CN for intermediate and high-risk mRCC patients [Citation5]. Also, the randomized SURTIME study designed to evaluate the timing of the systemic therapy, accrued poorly and the results must be regarded as mainly exploratory [Citation6]. In that study, an overall survival (OS) benefit was suggested in favor of the deferred CN arm. Further, based on Metastatic Renal Cancer Database Consortium (IMDC) data, CN was not recommended in patients with limited expected survival or those with four or more IMDC prognostic factors [Citation7]. These studies have substantially changed the guidelines recommendations and discussions in multidisciplinary conferences, generally in favor of deferred or no CN for most patients with mRCC. It remains unclear whether these recommendations are valid for the vast majority of mRCC patients.
Since January 2005, all patients diagnosed with RCC in Sweden are registered in the National Swedish Kidney Cancer Register (NSKCR). The coverage of the NSKCR is 99% of all patients with RCC in Sweden as compared to the Swedish Cancer Register to which reporting of all new cancer patients is mandated by law [Citation8,Citation9].
The aim of the study was to evaluate the long-term overall survival of patients with mRCC in relation to the first line treatment given, in a large material of unselected patients, based on a nation-wide population-based cohort.
Materials and methods
Patients
In the NSKCR from 2005 until 2013, 1483 (21.8%) of 6798 patients (951 males, 532 females) were identified with metastatic RCC (mRCC), at primary diagnosis. Their mean age was 66.8 years in men (range 22–91 years) and 70.0 years in women (range 33–105 years) (). The patient’s personal identity number were linked to the Swedish National Population Register for overall survival information. Survival time was defined as the time from diagnosis to date of death of any cause or alive at the end of July 2019. Information about the patient’s primary treatments were registered in the NSKCR, as well as TNM stage, RCC type, tumor grade and tumor size, patient’s age and sex. Linkage to individual patients’ identification was deleted before statistical analysis. Patients were categorized according to primary treatment given after diagnosis of mRCC, as follows: surgical treatment (nephrectomy, partial nephrectomy, tumor ablation) or systemic medical or palliative treatments. There was no data available on second line therapy, as nephrectomy after systemic therapy or treatment with systemic therapy after nephrectomy in the NSKCR. Furthermore, no data on metastatic sites or metastatic burden was available. Most patients with metastatic disease had been discussed at a multidisciplinary therapy conference before the treatment recommendation.
Table 1. Patients age, tumor size, gender, and distribution of T-stage, N-stage, Fuhrman grade and RCC type are shown in relation to given primary treatment in 1483 patients with metastatic renal cell carcinoma at diagnosis. Percentages of gender distribution in brackets.
Statistical analysis
Mann-Whitney U and Chi-square tests were performed for statistical calculations. Patient’s overall survival (OS) was estimated by the Kaplan-Meyer method and analyzed by the Log-rank test. Multivariate analyses using Cox regression models were carried out to identify potential independent prognostic information. Thirty days mortality was determined from date of surgery, or start of treatment. A two-tailed p value of <.05 was considered statistically significant. Statistical analysis was performed using the SPSS version 26.
Results
Surgery was the most common primary treatment (835 patients) including 804 patients with radical nephrectomy, 21 with partial nephrectomy and 10 with ablative treatment. First line systemic therapy was offered to 279 patients and palliation only, to 369 patients.
Men were more frequently treated surgically, while women more commonly were offered palliation (p = .002, and p < .001 respectively, ). Women were significantly older than men while the distribution of T-Stage was similar between genders ( and ). As shown in , patients primary treated with surgery and systemic treatment were significantly younger than those treated with palliation. Patients with upfront surgery had larger tumors than both patients primary treated with systemic therapy or palliation (p < .001 and p = .001, respectively, ).
Table 2. Distribution of T-stages in relation to gender in 1483 patients with metastatic renal cell carcinoma.
There was a significantly longer (p < .001) overall survival (OS) in patients treated with upfront CN than in patients primarily treated with systemic therapy or palliation. This OS difference was found in all age groups, as shown in . In univariate analysis, including: given primary treatment, T-stage, lymph node spread and patient age, all predicted OS. In a multivariate Cox regression analysis, primary treatment with no surgery, positive lymph nodes, higher T-stage, and higher patient age remained as independent negative predictors of OS, while year of treatment independently associated to longer OS. (). The mean and median survival times, in relation to primary therapy, are illustrated in . The 5-year survival rates in patients treated with primary surgery, systemic therapy and palliation were 22.0% 3.9% and 3.0% respectively. The corresponding10-year survival rates were 7.2%, 0.4% and 0.3%, respectively. The 30 days mortality rate was 1.6% for surgically treated patients, 2.5% in patients with first line systemic therapy and 14.6% in those offered palliation.
Figure 1. Kaplan-Meyer survival curves illustrating overall survival in relation to primary treatment in mRCC patients younger than 60 years, showing significant survival differences between all given treatments (p < .001).
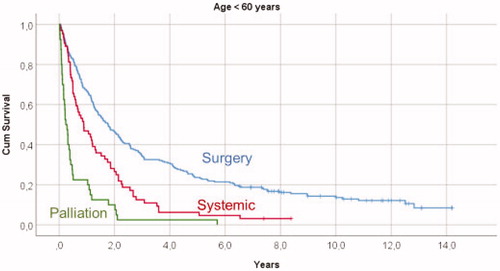
Figure 2. Kaplan-Meyer survival curves illustrating overall survival in relation to primary treatment in mRCC patients 60–80 years old, showing significant survival differences (p < .001) when comparing surgical treatment with both systemic therapy and palliation, and a significant survival difference (p = .001) between systemic therapy and palliation.
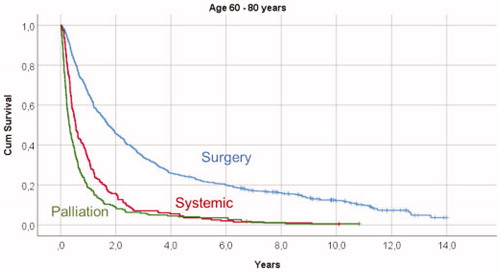
Figure 3. Kaplan-Meyer survival curves illustrating overall survival in relation to primary treatment in mRCC patients older than 80 years, showing significant survival differences between the given treatments (p < .001) when comparing surgical treatment with both systemic therapy and palliative treatments, but no difference in overall survival (p = .981) between patients treated with systemic therapy and palliation.
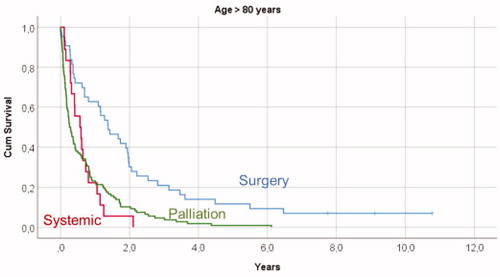
Table 3. Hazard ratio (HR) and 95 % confidence interval for overall survival in 1483 patients with metastatic renal cell carcinoma at primary diagnosis according to: T-stage, tumor size, N-stage, given treatment, gender, and patients age groups and years of diagnosis, using a multivariate Cox regression analysis.
Table 4. Mean and median overall survival times, in years, with 95% confidence intervals, are shown in relation to the given primary therapy in 1483 patients with metastatic renal cell carcinoma.
Discussion
The best primary treatment of patients with mRCC at diagnosis remains controversial. The present study in the early TKI era, suggests that in a national cohort, patients with primary surgery had a superior OS than those with primary systemic therapy. Patients with first-line CN had a significant overall survival advantage in all age groups. The selection for the primary treatment was done in the real word situation, possibly based on the performance status of the patients and the preferences by the multidisciplinary team. All patients in the present study would be classified as IMDC intermediate or high-risk patients, since all had synchronous metastatic disease [Citation7]. Thus, after clinical selection of patients, mostly after a multidisciplinary therapy conference, we found significantly longer OS for patients primarily treated with CN, compared with both primary systemic treatment and palliation. Women underwent CN less frequently than men. The reason for this difference might have been a significantly higher age at diagnosis for women with mRCC, while there was no obvious difference in the distribution of TNM stage between the genders. CN was more frequently performed in younger patients, probably due to clinical selection. The mean size of the primary tumor was significantly larger in patients treated with CN than in patients treated with systemic therapy or palliation. It was previously reported that mRCC patients benefit from CN if they have the following factors: a good performance status, metastases limited to one organ, low ESR, normal serum calcium and no tumor thrombus [Citation10]. Patients with these criteria commonly were recommended primary CN, a selection influencing the outcome in the present study.
Our results are supported by previous randomized studies comparing interferon-α and CN versus interferon-α only showing a limited but significant survival advantage for CN [Citation2]. Several hypotheses have been suggested to explain the better survival after the combination of CN and interferon-α, such as removal of immunological blocks and reduced production of growth factors and cytokines by the primary tumor in situ [Citation11,Citation12]. Also, a reduced metastatic potential might be involved. After the introduction of VEGFR-targeted therapy (TKI) there was more than two years benefit for mRCC patients with intermediate prognostic risk who underwent several treatment TKI lines. Most of these patients had primarily been treated with CN [Citation1]. Recently a systematic review of 56 studies evaluating effects of CN demonstrated an OS advantage of CN in patients with mRCC in the ten retrospective comparative trials with less selection bias than the other 46 studies [Citation13]. However, also these ten studies were biased towards a more favorable population undergoing CN than in those not undergoing CN [Citation13]. In another recent review the authors concluded that the best available tools to aid in the selection of primary therapy are the number of clinical prognostic risk factors, number of surgical risk factors, site and number of metastases, PS, and tumor disease burden [Citation14].
The results of the recently published randomized CARMENA and SURTIME studies, both prematurely closed, indicated that patients with mRCC having intermediate and high risk should be treated with primary systemic therapy instead of primary CN [Citation5,Citation6]. The CARMENA phase III study, which compared immediate CN followed by sunitinib versus sunitinib alone, showed that sunitinib only was not inferior to CN followed by sunitinib with regard to OS. That trial included 450 out of planned accrual of 576 patients with metastatic ccRCC of poor and intermediate MSKCC risks with a median follow-up of 50.9 months. In the intention to treat analysis, the median OS in the CN-sunitinib group was 13.9 months vs. 18.4 months in the sunitinib only group. Despite this 4.5-month difference in OS in favor of the sunitinib only group, this difference was non-significant. In the present study all patients had at least six years potential follow-up time. Patients primarily treated with CN in our study, including MSKCC intermediate and high-risk groups, had a median OS of 20.6 months (mean 42 months) compared with median OS of 13.9 months in CARMENA-CN patients and 19.0 months for intermediate-risk patients. In contrast to the median OS of 18.4 months in the CARMENA sunitinib only arm our patients primarily treated with first-line systemic therapy, had a median OS of 7.2 months. However, similar OS times (8.3 months) as in the present study was reported in patients who did not undergo surgery but were treated with sunitinib alone in the CARMENA intention to treat CN arm. Of note, 38 patients (17%) in the sunitinib only arm required secondary CN due to acute symptoms or for complete or near-complete response. The median time from randomization to second line treatment with CN was 11.1 months. This shows that secondary CN was performed in at least 30% of the CARMENA sunitinib patients who survived longer. This indicates a need for CN after sunitinib therapy also suggesting that deferred CN, in patients with response or stable disease after TKI, offers a survival benefit [Citation15]. The randomized SURTIME, study, comprising only 99 of 458 intended patients, investigated whether immediate versus deferred CN combined with sunitinib, could identify patients therapy resistant to sunitinib [Citation6]. The findings suggest that a deferred CN approach in patients who start treatment with sunitinib and are offered CN only if their disease does not progress, might be superior to performing CN up front followed by sunitinib therapy. In the SURTIME study the median OS in the immediate CN group was 15.0 months and 32.4 months in the deferred arm. These data were comparable to survival data of other single-arm phase 2 studies of presurgical sunitinib (26.0 months) and pazopanib (22.7 months) [Citation16,Citation17]. However, in the SURTIME study the progression free survival curves were similar in both groups during the entire follow-up, and after exclusion of ineligible patients, the OS Kaplan-Meyer curves were comparable between the groups [Citation6]. The 1.6%, surgical 30 days mortality rate in the present study, influenced OS less than the in-house mortality rate of 4.3% in the immediate CN arm in the SURTIME study and 2.0% in the CARMENA study. The in-house mortality is reported up to 3.6% in other CN studies [Citation18]. Thus, our results with a low surgical mortality in the national cohort indicate that CN rarely impede the possibility of second-line therapy.
Our real-world results, in unselected patients with mRCC, showed that the outcome after primary CN, was superior to first-line systemic therapy, in the early TKI era, based on clinical and multidisciplinary judgement. Our unselected national cohort also showed low 30 days mortality rates in patients with up-front CN. On the other hand, this register study also showed that the survival was somewhat improved in the later study years supporting better effect of the systemic therapy in first and later treatment lines, possibly due to more experience of TKI treatment with time in patients with mRCC [Citation19,Citation20]. The recent advances in immunotherapy will possibly change the options for CN. The new treatment for patients with intermediate- and poor-risk mRCC with superiority of nivolumab and ipilimumab over sunitinib, and pembrolizumab and axitinib over sunitinib in survival and quality of life, in the front-line setting, might change the treatment options for primary CN accordingly. Immunotherapy also has a potential to offer surgery with minimal delay in medical systemic treatment [Citation4,Citation21,Citation22].
This study has several limitations besides being retrospective and register based. The selection of the primary treatment based on performance status and treatment preferences is a major risk for biases of the results. Other major limitations were that the register did not include data on metastatic burden, number of, or location of metastatic sites and included no data on which systemic therapy was given, as primary or as second line therapy after nephrectomy. Furthermore, there were no data on performance status, surgical risk factors, or oncologic eligibility criteria. It is obvious that the selection of the primary therapy has been based on these factors.
It can be assumed that only a minor subset of patients will have a solitary or oligometastatic disease, while the vast majority of patients with mRCC have a multiorgan metastatic disease [Citation1]. Most of the patients primarily treated with upfront CN would have been subjects for a later systemic therapy. In patients primarily treated with systemic therapy, there was no specified data on the first-line systemic treatment used. In addition, the register had no data on any second-line therapy, neither systemic therapy after nephrectomy nor deferred nephrectomy after systemic therapy. Despite these limitations, our results seem meaningful, for the evaluation of the outcome of the primary first-line treatment decisions in patients with mRCC. A significant advantage of the study is that it comprises an unselected large patient cohort that covers all patients nationwide and shows real-world results. Also, the data on OS is valid since it is based on the linkage to the Swedish National Population Register that covers information of all individuals in the country.
Conclusions
The present study, based on a national large cohort of patients with metastatic RCC and a long follow-up, showed that patients selected for primary CN, had a significant overall survival advantage in all age groups. In multivariate analysis, the offered primary treatment, the occurrence of lymph node metastases, and patient age remained as independent predictors of OS. The results showed that CN is still an essential option for first-line treatment of patients with mRCC.
Ethical approval
The study was performed after approval of the regional Ethical Review Board of northern Sweden (Dnr 2012-418-31 M).
Acknowledgements
This project was made possible by the continuous work of the National Swedish Kidney Cancer Register of Sweden steering group: Börje Ljungberg (chairman), Peter Elfving, Marcus Thomasson, Britt-Inger Kröger Dahlin, Annika Håkansson, Åsa Jellvert, Pernilla Sundqvist, Benny Holmström, Per Lindblad, Magnus Lindskog, Ulrika Harmenberg, Ann-Helén Scherman-Plogell, Anders Kjellman, Andreas Thorstenson, Per Skoglund, Linn Pettersson, Emma Ulvskog, Magnus Fovaeus, Sven Lundstam, Mikael Hellström, Jörgen Jehander, and Martin Johansson. The authors also thank Soheila Hosseinnia and other collaborators at the Regional Cancer Centre, Stockholm, for providing data from the NSKC.
Disclosure statement
Börje Ljungberg: Company speaker honorarium: Novartis, Pfizer, IPSEN, BMS, Trial participation: Jenssen - Astellas - Medivation, Company consultant: Janssen, Ipsen, MSD; Other: EAU;, Pernilla Sundqvist No conflict of interest;, Per Lindblad No conflict of interest;, Anders Kjellman; Company speaker honorarium: Astellas, Janssen, Advisory board Astellas, Trial participation Bayer, Janssen;, Andreas Thorstenson: No conflict of interest;, Mikael Hellström: No conflict of interest;, Britt-Inger Kröger Dahlin: No conflict of interest;, Marcus Thomasson: Advisory board, Ibsen;, Ulrika Harmenberg Company speaker honorarium: Pfizer, BMS,IPSEN, Advisory boards: BMS, IPSEN;, Sven Lundstam: Advisory board MSD, BMS, Ipsen.
References
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810.
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171(3):1071–1076.
- Bex A, Albiges L, Ljungberg B, et al. Updated European association of urology guidelines for cytoreductive nephrectomy in patients with synchronous metastatic clear-cell renal cell carcinoma. Eur Urol. 2018;74(6):805–809.
- Culp SH. Cytoreductive nephrectomy and its role in the present-day period of targeted therapy. Ther Adv Urol. 2015;7(5):275–285.
- Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–427.
- Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5(2):164–170.
- Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014;66(4):704–710.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34(8):1081–1086.
- Thorstenson A, Harmenberg U, Lindblad P, et al. Impact of quality indicators on adherence to National and European guidelines for renal cell carcinoma. Scand J Urol. 2016;50(1):2–8.
- Ljungberg B, Landberg G, Iranparvar Alamdari F. Factors of importance for prediction of survival in patients with metastatic renal cell carcinoma, treated with or without nephrectomy. Scand J Urol Nephrol. 2000;34(4):246–251.
- Lahn M, Fisch P, Köhler G, et al. Pro-inflammatory and T cell inhibitory cytokines are secreted at high levels in tumor cell cultures of human renal cell carcinoma. Eur Urol. 1999;35(1):70–80.
- Xia Y, Zhang Q, Zhen Q, et al. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget. 2017;8(23):37783–37795.
- Bhindi B, Abel EJ, Albiges L, et al. Systematic review of the role of cytoreductive nephrectomy in the targeted therapy era and beyond: an individualized approach to metastatic renal cell carcinoma. Eur Urol. 2019;75(1):111–128.
- Soares A, Maia MC, Vidigal F, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma: how to apply new evidence in clinical practice. Oncology. 2020;98(1):1–9.
- Bhindi B, Graham J, Wells JC, et al. Deferred cytoreductive nephrectomy in patients with newly diagnosed metastatic renal cell carcinoma. Eur Urol. 2020;S0302-2838(20)30301-8. doi: 10.1016/j.eururo.2020.04.038.
- Powles T, Blank C, Chowdhury S, et al. The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. Eur Urol. 2011;60(3):448–454.
- Powles T, Sarwar N, Stockdale A, et al. Safety and efficacy of pazopanib therapy prior to planned nephrectomy in metastatic clear cell renal cancer. JAMA Oncol. 2016;2(10):1303–1309.
- Wallis CJ, Bjarnason G, Byrne J, et al. Morbidity and mortality of radical nephrectomy for patients with disseminated cancer: an analysis of the National Surgical Quality Improvement Program database. Urology. 2016;95:95–102.
- Lindskog M, Wahlgren T, Sandin R, et al. Overall survival in Swedish patients with renal cell carcinoma treated in the period 2002 to 2012: update of the RENCOMP study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35(9):541.e15–541.e22.
- Beisland C, Johannesen TB, Klepp O, et al. Overall survival in renal cell carcinoma after introduction of targeted therapies: a Norwegian population-based study. Onco Targets Ther. 2017;10:371–385.
- Motzer RJ, Tannir NM, McDermott DF, et al. CheckMate 214 investigators. nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290.
- Psutka SP, Chang SL, Cahn D, et al. Reassessing the role of cytoreductive nephrectomy for metastatic renal cell carcinoma in 2019. Am Soc Clin Oncol Educ Book. 2019;39:276–283.
