Abstract
Objective
To investigate time spent in hormone-sensitive and castration-resistant disease states in men with advanced prostate cancer in Sweden, and the associated health economic impact.
Materials and methods
Registry study (NCT03619980) of the Prostate Cancer data Base Sweden with data from the National Prostate Cancer Register, including the Patient-overview Prostate Cancer (PPC) and other national healthcare registries. The primary endpoint was time in each disease state. Secondary endpoints were co-medications, comorbidities and healthcare resource utilization (HRU) and cost in each disease state.
Results
In total, 1,869 men with advanced prostate cancer registered in PPC between 2014 and 2016, with data on the start of androgen deprivation therapy, were identified. Median time to progression and median survival were 4 and 11 years, respectively, for men with non-metastatic (nm) hormone-sensitive prostate cancer (HSPC); 1 and 7 years for men with metastatic (m) HSPC; and 1 and 8.5 years for men with nm castration-resistant prostate cancer (CRPC). Median survival for men with mCRPC was 4 years. Total annual mean costs for HRU per patient increased with increasing severity of disease, from 41,064 Swedish krona (SEK) for nmHSPC to 288,242 SEK for mCRPC.
Conclusion
Progression time from mHSPC and nmCRPC to the mCRPC state was short and survival in the mCRPC state was approximately 4 years. Survival times were longer than expected, likely due to the selection of long-term survivors among prevalent cases. Healthcare costs were high for men with mCRPC. Further studies are needed to confirm our pilot study findings.
Introduction
Prostate cancer is the most common cancer among men in Nordic countries, with approximately 24,000 men diagnosed each year [Citation1,Citation2]. In parallel with a strong increase in prostate cancer incidence since the 1990s, there has been a modest decline in prostate cancer mortality during the same time period [Citation1]. This, combined with an aging population, has resulted in a rapid increase in prostate cancer prevalence.
Most men who are diagnosed with prostate cancer have localized disease, some of whom subsequently start androgen deprivation therapy (ADT) due to cancer progression. Approximately 15% of men with prostate cancer have metastasis at diagnosis, and these men have short survival [Citation3]. Since the 1940s, ADT had been the mainstay treatment for metastatic (m) prostate cancer [Citation4]. After a variable amount of time on ADT, disease progression reaches the castration-resistant prostate cancer (CRPC) state [Citation4,Citation5]. In Sweden, 2,400 men die from prostate cancer each year, and, in all likelihood, a large majority of these men have transitioned through the mCRPC state [Citation6].
In recent years, several life-prolonging treatments have been introduced for men with advanced prostate cancer. Randomized trials have demonstrated prolonged survival and improved quality-of-life in men with non-metastatic (nm) CRPC, mCRPC and, recently, also in men with metastatic hormone-sensitive prostate cancer (HSPC; also referred to as castration-sensitive prostate cancer) [Citation4,Citation7]. The uptake of these treatments has been slow, with large regional differences, where high treatment costs may have contributed to these differences [Citation8,Citation9]. Little is known about how long men spend in each prostate cancer disease state and the subsequent effect this has on healthcare resource utilization (HRU) and costs.
To address this lack of information, we aimed to assess the time spent in each disease state in men with advanced prostate cancer and the associated HRU and costs using real-world data from the Patient-overview Prostate Cancer (PPC), a novel part of the National Prostate Cancer Register (NPCR) of Sweden.
Materials and methods
Setting and data sources
This non-interventional, registry study was conducted at the Regional Cancer Centre, Uppsala Örebro, Sweden. Data were extracted from the Prostate Cancer data Base Sweden (PCBaSe), a research database that was created by linking data in NPCR, including its primary registration and the novel PPC, with data from other national healthcare registries and demographic databases [Citation10]. These include The Prescribed Drug Registry, The National Patient Registry, Cause of Death Registry, and the longitudinal integration database for health insurance and labor market studies (LISA socioeconomic database).
Since 1998, the primary registration in NPCR captures 98% of all men diagnosed with prostate cancer in The Cancer Registry, to which registration is mandated by law. NPCR currently holds data on primary treatment and work-up for > 180,000 men diagnosed with prostate cancer in Sweden.
PPC is a novel part of NPCR, initiated in 2014, which contains longitudinally registered data from men with advanced prostate cancer [Citation11]. PPC was initiated to overcome the main shortcoming of the primary registration in NPCR, which is lack of follow-up data on prostate-specific antigen (PSA) progression, imaging, and treatments other than those registered in The Prescribed Drug Registry, such as chemotherapy and radionuclide treatment, beyond the period of primary treatment and work-up. These data are not captured by linkages to The National Patient Registry or The Prescribed Drug Registry in PCBaSe. Men who start ADT for prostate cancer are eligible to be registered in PPC and, for men who have already reached the CRPC state at the time of inclusion in PPC, selected data from the date of ADT initiation are retrieved from medical records and entered into PPC.
This study was approved by the Regional Ethics Review Board in Uppsala and is registered with ClinicalTrials.gov (NCT03619980).
Study population
The study population comprised men registered in PPC between 1 January 2014 and 31 December 2016. For these men, prospective data were available from the date of registry entry until death or 31 December 2016, and data related to events prior to their entry into PPC were extracted from medical records going back no further than 1 January 2006. Prostate cancer disease states are defined in . Men were excluded from the study if they did not have a start date for ADT initiation in PPC, if the start date in PPC did not coincide with that of a filled prescription for an ADT in The Prescribed Drug Registry, or if they had started ADT before 2006.
Table 1. Definition of prostate cancer disease states.
The primary endpoint was time from date of entry into each prostate cancer disease state to date of progression to a new disease state or death. Secondary endpoints included co-medications, comorbidities, HRU, and cost for each disease state. HRU was measured by number and type of prostate cancer treatment regimens, frequency and duration of hospitalization, type and frequency of out-patient visits, frequency of imaging, and frequency of laboratory tests (PSA and alkaline phosphatase).
Three sensitivity analyses of the primary endpoint were conducted. The first analysis used an alternative definition of CRPC, based on PSA progression according to the PPC registry manual and Swedish National Guidelines for prostate cancer (). A temporal sensitivity analysis was also performed, restricted to men who started ADT after 1 January 2013. The third sensitivity analysis was performed in men with mCRPC, stratified by the presence or absence of metastases at the date of prostate cancer diagnosis, based on imaging registered in NPCR. This was performed to assess whether the disease trajectory prior to entry into the mCRPC state affected the duration of time in the mCRPC state.
Statistical analyses
This was a descriptive study and did not include an a priori hypothesis or any power calculations. No formal statistical comparisons between any groups were planned.
The study group included men registered in PPC between 1 January 2014 and 31 December 2016 who had a first record of lifelong ADT within the study period (2006 − 2016). Time to progression was estimated using competing risk analysis of cumulative incidence of progression or death and overall survival was estimated using Kaplan–Meier analysis. Unit prices from official sources were used for cost calculations, which are presented as annual cost per patient (2018 Swedish krona [SEK]). HRU was assessed by use of NordDRG weights [Citation12], with additional information taken from Southern and South-Eastern healthcare regions pricelists [Citation13–15]. Pharmaceutical costs, when not available in the Prescribed Drug Registry, were estimated based on information from The Dental and Pharmaceutical Benefits Agency (www.tlv.se) and www.apoteket.se.
Results
Patient characteristics
Of the 2,435 eligible men registered in PPC up to 31 December 2016, 566 men were excluded, resulting in a study group of 1,869 men with prostate cancer. Reasons for exclusion were: no ADT initiation date (n = 307); not registered in primary registration of NPCR (n = 115); ADT initiation prior to 2006 (n = 100); discrepancy in ADT start date between PPC and The Prescribed Drug Registry (n = 37); and no confirmation of ADT initiation date (n = 7). At the start of ADT, 79% of men were aged ≥ 66 years and 3% were aged ≤ 55 years. At prostate cancer diagnosis, 32% of men had metastases. shows the characteristics of men by disease state. Mean age at entry into disease state ranged from 70 years for mHSPC to 75 years for nmCRPC, and mean time from the date of prostate cancer diagnosis to entry into the disease stage ranged from 1 year for mHSPC to 5 years for nmCRPC.
Table 2. Patient characteristics by disease state at date of entry into disease state for men registered in PPC.
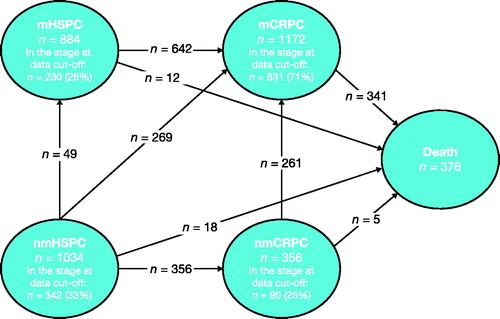
Disease state transitions are illustrated in . Slightly more than one-half of men in the mCRPC state had made a transition from the mHSPC state, whereas roughly one-quarter had made a transition from the nmHSPC state, and another one-quarter from the nmCRPC state. Over 90% of men who died had made the transition through the mCRPC state.
Primary endpoint
Men in the nmHSPC state had an almost 3-fold longer time to progression than men in the mHSPC and nmCRPC states. Median time to progression and median survival were 4 and 11 years, respectively, for men in the nmHSPC state, 1 and 7 years for men in the mHSPC state, and 1 and 8.5 years for men in the nmCRPC state. Median time to death, the only possible transition for men in the mCRPC state, was 4 years ( and , and Supplementary Table S1).
Figure 2. Time from entry into disease state to progression or death. A man can be included in more than one disease state due to transitioning between states. Stacked cumulative incidence analysis. CRPC, castration-resistant prostate cancer; HSPC, hormone-sensitive prostate cancer; m, metastatic; nm, non-metastatic.
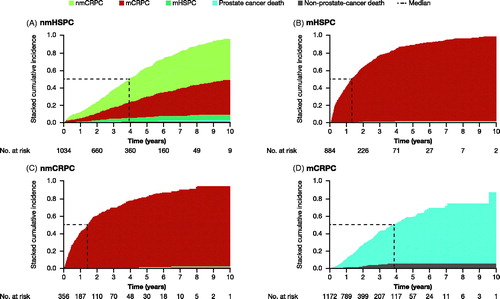
Figure 3. Time from entry into disease state to death from any cause. A man can be included in more than one disease state due to transitioning between states. Kaplan–Meier analysis. CI, confidence interval; CRPC, castration-resistant prostate cancer; HSPC, hormone-sensitive prostate cancer; m, metastatic; nm, non-metastatic.
Sensitivity analyses based on an alternative definition of CRPC, according to PSA increases, resulted in a decrease in the number of men in the nmCRPC and mCRPC states and ensuing slight decreases in median time to progression and death in men with CRPC, while median time to progression increased slightly in men with HSPC with no change in median time to death (Supplementary Table S2). Temporal sensitivity analyses, restricted to 991 men who started ADT after 1 January 2013, showed that the median time to progression was shorter for men with HSPC and similar for men in the nmCRPC state compared to the full study group. Time to death was shorter for men in the nmCRPC and mCRPC states, compared to that for the full study group (Supplementary Table S3). There were too few events for men with nmHSPC or mHSPC in this time frame to reach a median survival estimate. The majority of men with mCRPC did not have metastases at the date of diagnosis (61%) and, in an analysis based on the presence or absence of metastases at date of diagnosis, we found no difference between these two groups in median time from date of entry into the mCRPC state to death (Supplementary Table S4).
Secondary endpoints
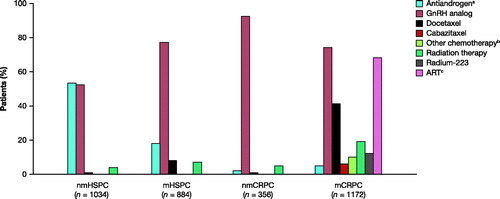
Among men with HSPC, regardless of metastatic status, the most common prostate cancer treatments were ADT, by use of antiandrogens or gonadotropin-releasing hormone (GnRH) analogs. Men in the nmCRPC state were almost exclusively treated with a GnRH analog (92%), while those in the mCRPC state received multiple different treatments, with GnRH analogs (74%), androgen receptor-targeted therapies (68%), and docetaxel (41%) among the most common ().
Figure 4. Prostate cancer treatments by disease state. A man can be included in more than one disease state due to transitioning between states. Men may have received more than one treatment. aAntiandrogen includes: bicalutamide; bOther chemotherapy includes: cisplatin, cyclophosphamide, estramustine, etoposide gemcitabine, carboplatin, methotrexate or mitoxantrone; cART includes: enzalutamide or abiraterone. ART, androgen receptor-targeted therapy; CRPC, castration-resistant prostate cancer; GnRH, gonadotropin-releasing hormone; HSPC, hormone-sensitive prostate cancer; m, metastatic; nm, non-metastatic.
Co-medications received in the year prior to disease state entry are reported in Supplementary Table S5. Overall, the most common co-medications were analgesics and antipyretics, antithrombotic agents, and opioids, with an increased usage in more advanced disease states, particularly in mCRPC.
The presence of comorbidities was similar among all disease states (Supplementary Table S6). The most common comorbidities in each disease state were diabetes, myocardial infarction, congestive heart failure, and cerebrovascular disease.
HRU is presented in Supplementary Table S7. There were few hospitalizations with prostate cancer as the primary diagnosis in any disease state; however, the incidence was highest in men in the mCRPC state, for whom the duration of hospitalization with prostate cancer as the primary diagnosis was the longest. Men in the mCRPC state were also most likely to be hospitalized for any reason; only 40% of these men had no hospitalizations, vs 61%, 68% and 69% of men in the nmHSPC, mHSPC and nmCRPC states, respectively. Accordingly, the number of outpatient physician visits, imaging procedures and laboratory tests was higher in men in a more advanced disease state.
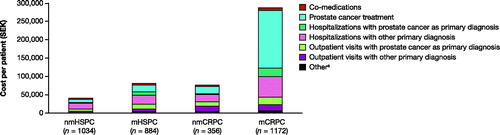
Total mean costs for HRU per patient per year increased from 41,064 SEK in the nmHSPC state, 81,072 SEK in the mHSPC state and 76,667 SEK in the nmCRPC state to 288,242 SEK in the mCRPC state, with prostate cancer treatments comprising the largest portion (). For men in the mCRPC state, these treatments made up 55% of total HRU costs, whereas for men in other disease states it was no larger than 26% of total HRU costs.
Figure 5. Annual mean HRU costs per patient by disease state. A man can be included in more than one disease state due to transitioning between states. Costs are based on list prices and are provided in SEK 2018. aIncludes nurse visits, telephone consultations, imaging examinations, and laboratory tests (PSA and ALP). n = 363, n = 282, n = 112, and n = 1,084 for nmHSPC, mHSPC, nmCRPC, and mCRPC, respectively. ALP, alkaline phosphatase; CRPC, castration-resistant prostate cancer; HSPC, hormone-sensitive prostate cancer; m, metastatic; nm, non-metastatic; PSA, prostate-specific antigen; SEK, Swedish krona.
Discussion
Using real-world data from over 1,800 men with advanced prostate cancer from a clinical cancer register, including prevalent cases, we analyzed the time spent in each disease state and the associated HRU and costs. Our findings suggest that time to progression was approximately 4 years for men in the nmHSPC state, with an annual cost for HRU of 41,064 SEK. For men in the mHSPC state, time to progression was approximately 1 year and HRU costs were 81,072 SEK. Time to progression was approximately 1 year for men in the nmCRPC state, with associated HRU costs of 76,667 SEK. In men in the mCRPC state, time to death was approximately 4 years and HRU was highest at 288,242 SEK.
HRU increased with increasing severity of disease and total annual mean cost per patient increased 7-fold from the nmHSPC state (41,064 SEK) to the mCRPC state (288,242 SEK). Differences in prostate cancer treatments, including increased use of chemotherapy (mostly docetaxel) and androgen receptor-targeted drugs was the main driver of cost in the mCRPC state, but an increase in hospitalizations also contributed. As only limited prospective data were available, analysis of HRU related to nurse visits, telephone consultations, imaging and laboratory tests were based on a smaller sample size and may be affected by missing data in PPC. Lack of comparable studies in terms of design, population and date of analysis makes validation of our findings and comparison to the existing literature challenging [Citation16].
Our analyses have some inherent limitations. This was a pilot study in PPC, a novel part of NPCR, which captured 36% of men in NPCR who had filled a prescription for androgen receptor-targeted therapy (abiraterone or enzalutamide) [Citation11]. The major limitation of our study was the inclusion of both prevalent and incident cases in PPC. This entailed selection of long-term survivors among prevalent cases, resulting in an exaggeration of the survival times in each disease state. We observed a median survival of 7 years in men in the mHSPC state; however, in a recent population-based study of men with de novo metastatic prostate cancer in NPCR (i.e. 95% of all men with prostate cancer in Sweden), median survival was found to be around 3 years [Citation3]. Recent randomized trials in men with mHSPC have reported survival times of 4–5 years in the experimental arms and 3–4 years in the control arms [Citation17–19]. Similarly, we report a median survival of approximately 4 years in men in the mCRPC state; however other population-based cohort studies have reported survival times of 1–3 years [Citation20–22]. In order to minimize the influence of selection for long-term survivors, we performed a temporal sensitivity analysis, which was restricted to men who started ADT after 1 January 2013. Survival times were found to be shorter and closer to the survival times reported in the literature. Shorter survival times are expected when excluding long-term survivors and may, therefore, be conservative. Additional analyses on an incident cohort with longer follow-up are required to determine whether the mix of prevalent and incident cases in this sample biased the results. Other limitations of our study are that approximately 20% of eligible men in PPC who met the inclusion criteria were excluded due to missing data and that a large proportion of men transitioned from nmHSPC to mCRPC, i.e. hormone-sensitive men with no metastases who directly transitioned to a state with both metastases and castration resistance. The latter may relate to lack of regular imaging during routine follow-up in these men, in combination with the potential for missing data in PPC.
Nonetheless, a key strength of this study is the use of a real-life data set, outside of a randomized controlled trial. This provides comprehensive data from a longitudinal registration of prostate cancer-specific variables in addition to data from a number of nationwide, population-based healthcare registers with known high-quality data [Citation23,Citation24]. No more than 2% of men in the nmHSPC, mHSPC, and nmCRPC states died of prostate cancer; it is likely that these men had missing data in PPC that precluded a transition to the mCRPC state, and that all men who die of prostate cancer make a transition through the mCRPC state. Thus, although our results were somewhat affected by selection bias, we argue that even if the time spent in each disease state was inflated, our results suggest that many men will make a rapid transition to a CRPC state and will remain in these states for several years. To the best of our knowledge, there are few other studies that have attempted to assess the time spent in these disease states [Citation20–22]. With time, the proportion of incident cases will increase in PPC and this will improve the validity of future studies. Meanwhile, these results provide an approximation of the time spent in each prostate cancer state in men with advanced prostate cancer, and an indication of the cost of treatment of men with advanced prostate cancer.
A number of novel treatments have recently been shown to increase the survival and quality-of-life in men with metastatic disease, and more such drugs will soon become available [Citation4,Citation7]. Our results indicate that healthcare costs are related to disease state; therefore, with survival duration increasing in men with advanced prostate cancer, additional funding may be required in the future. Data from randomized trials are considered the gold standard for assessing the efficacy of drugs, but real-life data are required in order to assess whether results from randomized trials, which are often based on selected patient groups, are also attained in clinical practice where men are generally older and have more comorbidities. PPC was created to longitudinally collect data on men with advanced prostate cancer in Sweden. As PPC matures with longer follow-up and a larger number of patients, it will serve as an increasingly valuable resource for real-world data studies.
In conclusion, these real-world data from a clinical cancer register, including prevalent cases, suggest that time to progression was approximately 1 year for men in the mHSPC state, approximately 4 years for men in the nmHSPC state, and approximately 1 year for men in the nmCRPC state. In men in the mCRPC state, time to death was approximately 4 years. Survival times were longer than expected, likely due to selection of long-term survivors among prevalent cases. The annual healthcare cost increased substantially with increasing severity of disease from 41,064 SEK for men in the nmHSPC state to 288,242 SEK for men in the mCRPC state. Additional larger studies are needed to conclusively elucidate the time spent in each prostate cancer disease state.
isju_a_1851762_sm5098.pdf
Download PDF (138.2 KB)Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden steering group: Pär Stattin (Chair), Ingela Franck Lissbrant (Deputy Chair), Johan Styrke, Camilla Thellenberg Karlsson, Lennart Åström, Stefan Carlsson, Marie Hjälm-Eriksson, David Robinson, Mats Andén, Ola Bratt, Johan Stranne, Olof Akre, Per Fransson, Eva Johansson, Gert Malmberg, Hans Joelsson, Fredrik Sandin and Karin Hellström.
Disclosure statement
Johanna Svensson, Suzanne Kilany, and Karin Fagerlund are employed by Astellas Pharma a/s.
Region Uppsala has, on behalf of NPCR, made agreements on subscriptions for quarterly reports from PPC with Astellas, Sanofi, Janssen and Bayer, as well as joint research projects with Astellas, Bayer and Janssen. Ingela Franck Lissbrant has received speaker honoraria from AstraZeneca.
Data availability statement
Access to anonymized, individual, participant-level data will not be provided for this trial, as it meets one or more of the exceptions described on www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas.” The ethical approval limits the access to data.
Additional information
Funding
References
- NORDCAN. Cancer stat fact sheets; Nordic countries – Prostate; 2019 [cited 2019 Dec 16]. Available from: http://www-dep.iarc.fr/NORDCAN/english/StatsFact.asp?cancer=261&country=0.
- NORDCAN. Nordic countries (end of 2016). Prevalence, male, age 0–85+; 2019 [cited 2019 Dec 16]. Available from: http://www-dep.iarc.fr/NORDCAN/english/table13.asp?registry=0&period=2016&sex=1&stat=2&age_from=1&age_to=18&sort=2&submit=Execute.
- Westerberg M, Franck Lissbrant I, Damber JE, et al. Temporal changes in survival in men with de novo metastatic prostate cancer: nationwide population-based study. Acta Oncol. 2020;59(1):106–111.
- Cattrini C, Castro E, Lozano R, et al. Current treatment options for metastatic hormone-sensitive prostate cancer. Cancers. 2019;11(9):1355.
- Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32(49):5501–5511.
- NORDCAN. Cancer stat fact sheets; Sweden – Prostate; 2019 [cited 2019 Dec 16]. Available from: http://www-dep.iarc.fr/NORDCAN/english/StatsFact.asp?cancer=261&country=752.
- Moussa M, Papatsoris A, Abou Chakra M, et al. Pharmacotherapeutic strategies for castrate-resistant prostate cancer. Expert Opin Pharmacother. 2020;21(12):1431–1448.
- Franck Lissbrant I, Garmo H, Widmark A, et al. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013;52(8):1593–1601.
- Franck Lissbrant I, Ventimiglia E, Robinson D, et al. Nationwide population-based study on the use of novel antiandrogens in men with prostate cancer in Sweden. Scand J Urol. 2018;52(2):143–150.
- Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort Profile Update: The National Prostate Cancer Register of Sweden and Prostate Cancer Data base—a refined prostate cancer trajectory. Int J Epidemiol. 2016;45(1):73–82.
- Franck Lissbrant I, Hjälm Eriksson M, Lambe M, et al. Set-up and preliminary results from the Patient-overview Prostate Cancer. Longitudinal registration of treatment of advanced prostate cancer in the National Prostate Cancer Register of Sweden. Scand J Urol. 2020;54(3):227–234.
- Socialstyrelsen. Viktlistor för NordDRG; 2019 [cited 2019 Dec 16]. Available from: https://www.socialstyrelsen.se/utveckla-verksamhet/e-halsa/klassificering-och-koder/drg/viktlistor/.
- Region Skåne. Avgifter och prislistor; 2019 [cited 2019 Dec 16]. Available from: https://vardgivare.skane.se/patientadministration/avgifter-och-prislistor/.
- Södra sjukvårdsregionen. Regionala priser och ersättningar föregående år; 2019 [cited 2019 Dec 16]. Available from: https://sodrasjukvardsregionen.se/verksamhet/avtal-priser/regionala-priser-och-ersattningar-foregaende-ar/.
- Södra sjukvårdsregionen. Prislista för sydöstra sjukvårdsregionen; 2019 [cited 2019 Dec 16]. Available from: https://plus.rjl.se/infopage.jsf?nodeId=41089.
- Grochtdreis T, Konig HH, Dobruschkin A, et al. Cost-effectiveness analyses and cost analyses in castration-resistant prostate cancer: a systematic review. PLoS One. 2018;13(12):e0208063.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–1087.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30(12):1992–2003.
- Helgstrand JT, Røder MA, Klemann N, et al. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer—A population-based analysis of 2 national cohorts. Cancer. 2018;124(14):2931–2938.
- Bandini M, Pompe RS, Marchioni M, et al. Improved cancer-specific free survival and overall free survival in contemporary metastatic prostate cancer patients: a population-based study. Int Urol Nephrol. 2018;50(1):71–78.
- Aly M, Leval A, Schain F, et al. Survival in patients diagnosed with castration-resistant prostate cancer: a population-based observational study in Sweden. Scand J Urol. 2020;54(2):115–121.
- Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. 2015;51(1):101–111.
- Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol. 2015;54(2):158–163.