Abstract
Purpose
Intensified treatment such as extended lymph node dissection (LND) and/or perioperative chemotherapy in addition to radical nephroureterectomy (RNU) has been suggested for high-risk cases of upper tract urothelial carcinoma (UTUC). We aimed to identify preoperative predictors of tumour stage and prognosis in the diagnostic work-up before RNU. Further to evaluate if our findings could be used in selecting patients for intensified treatment.
Patients and methods
A total of 179 patients treated with RNU for UTUC at Haukeland University Hospital (HUS) and Vestfold Hospital Trust (VHT) during 2005–2017 were included in this retrospective study. All relevant preoperative variables regarding the patient, the CT and the ureteroscopy (URS) were registered and analysed regarding their ability to predict non-organ confined disease (NOCD, pT3+ and/or N+) at final pathology after RNU. The prognosis was assessed calculating survival for the cohort and stratified by preoperative variables.
Results
Local invasion and pathological lymph nodes at CT predicted NOCD in uni and multivariate regression analyses (OR 3.36, p=.004 and OR 6.21, p=.03, respectively). Reactive oedema surrounding the tumour (OR 2.55, p=.02), tumour size (4.8 vs. 3.9 cm, p=.006) and high-grade tumour at URS biopsy (OR 3.59, p=.04) predicted NOCD at univariate regression analyses. The 5-year CSS and OS for the entire cohort was 79% and 60%. ECOG, local invasion, pathological lymph nodes and reactive oedema surrounding the tumour at CT predicted CSS.
Conclusions
Several variables at the CT predicted both stage and survival. Local invasion at CT seems the most promising feature for selecting patients for intensified treatment.
Introduction
Urothelial carcinoma in the upper urinary tract (UTUC) is referred to constitute 5–10% of all urothelial carcinomas [Citation1]. UTUC is an aggressive disease and at diagnosis about 40% of the tumours are non-organ confined. The 5-year cancer-specific survival (CSS) in these cases is below 50% [Citation2,Citation3]. The standard treatment of invasive UTUC is a radical nephroureterectomy (RNU) with complete excision of the ipsilateral bladder cuff. Due to the high mortality of the disease, intensified treatment including chemotherapy as neo-adjuvant or adjuvant treatment or extended lymph node dissection (LND) have been suggested for high-risk patients [Citation4–7]. Due to the lack of accurate staging tools of the disease preoperatively, it can be challenging to identify the right indication for intensified treatment. Current EAU-guidelines recommend computed tomography (CT) urography as standard in diagnosis and preoperative staging of UTUC. A ureteroscopy (URS) is recommended if imaging and cytology are not sufficient for the diagnosis and/or risk-stratification of the tumour [Citation8].
The aim of this study was to analyse available preoperative factors regarding their ability to predict histopathological tumour stage and subsequent prognosis after RNU for UTUC in a contemporary cohort in Norway. We further sought to evaluate if our findings could be used in the selection of patients for intensified treatment.
Material and method
Patient selection
After obtaining approval from the Regional ethics committee (reference no. 2017/854), the medical records of 209 patients treated with a RNU between 2005 and 2017 for suspected UTUC at Haukeland University Hospital (HUS, n = 130) and the Vestfold Hospital Trust (VHT, n = 79), were retrospectively examined. A total of 30 patients were excluded due to concomitant bladder cancer with cystectomy in the same procedure as RNU (n = 9), non-urothelial cancer (n = 15, most of them renal cell carcinoma) or no malignancy detected at the final histopathological specimen after RNU (n = 6), leaving 179 patients for inclusion in the study.
Diagnostic work-up, treatment and follow-up
Standard preoperative assessment was a CT scan with contrast unless contraindicated. If there was doubt about the diagnosis or the patient was a potential candidate for kidney sparing treatment, a URS was performed. Endoscopic treatment or segmental ureter resection was considered among patients with low-stage UTUC of limited size clinically. The indication for RNU was a high-grade or invasive UTUC unless contraindicated due to comorbidity and/or high age. The RNU was performed as an open or laparoscopic procedure with complete excision of the bladder cuff. Chemotherapy was not standard treatment and neo-adjuvant chemotherapy was given to only one patient prior to RNU. LND was performed at the discretion of the surgeon. Follow-up included cystoscopy every three months for the first two years. A CT scan was commonly performed after 12 months or whenever the patient presented with symptoms suggestive of metastatic disease. Later follow-up was individualized.
Patient factors
Patient age, sex, comorbidities, kidney function, presenting symptoms and smoking status were registered together with the presence of prior bladder cancer or prior endoscopic treatment for UTUC.
Radiological analysis
A total of 176 (98%) of the patients were examined with a CT scan, 159 (90%) of these with a contrast-enhanced CT, 17 patients were examined with a CT without contrast due to kidney failure. One patient was examined with magnetic resonance imaging only, one with a conventional intravenous urography only and one lacked preoperative radiological examination of the upper urinary tract. All CT scans were re-evaluated by a uro-radiologist (LAR) together with a urologist (BA) and assessed regarding tumour size, location, contrast enhancement, the presence of hydronephrosis, pathological lymph nodes, reactive oedema surrounding the tumour and local invasion into renal parenchyma, the renal pelvis or periureteric tissue. Each variable was considered by the radiologist in each patient to assess if a reliable measurement could be made in that particular case. If for example reliable measurements regarding tumour size and/or contrast enhancement could not be made in one particular case, the variable was recorded as missing in the dataset. This results in a different number of patients available for analysis for each variable, as demonstrated in .
Table 3. Univariate analyses for prediction of non-organ confined disease according to variables at CT scan.
Ureteroscopy with biopsy and cytology evaluation
A total of 95 (53%) patients were examined with a preoperative URS with biopsy before RNU. A total of 60 patients were examined with a preoperative urinary cytology and 43 of these had cytology taken during URS. The ability of the biopsy to confirm UTUC diagnosis was registered together with information about biopsy tumour grade and stage.
Histopathological examination
Data regarding tumour location, stage and grade were gathered from the pathology reports at the respective institutions. Tumours were graded according to the two-tiered WHO 2004 classification [Citation9] and staged according to TNM 2017 classification [Citation10]. All specimens originally not concurring with these two classifications were re-examined and reclassified by uropathologists (OJH and BC).
Statistical analysis; prediction of prognosis and tumour stage
Continuous and categorical variables were analysed using a Student’s t-test and a chi-square test, respectively. Survival estimates were calculated using the Kaplan–Meier method, and a log-rank test was used to compare groups. The estimated 5- and 10 year overall survival (OS) and cancer (UTUC) specific survival (CSS) were calculated for the entire cohort. Furthermore, multivariate cox regression analyses including both patient features and final histopathology were performed to evaluate independent predictors of all-cause and cancer-specific mortality. The purpose of these analyses was to evaluate if survival and prognostic factors in the present cohort were similar to other larger published patient series on operated UTUC patients.
Univariate prediction of prognosis according to pure preoperative variables was then assessed by Kaplan–Meier estimates. Recurrence and metastasis after RNU for UTUC will in most cases result in death from UTUC, thus 5-year CSS was chosen as the primary outcome parameter. For prediction of tumour stage at final histopathological examination after RNU, all candidate variables regarding patient features, CT and ureteroscopic findings were analysed using univariate and multivariate logistic regression analysis to assess their abilities to predict non-organ confined disease (NOCD). NOCD was defined as pT3 or more (invasion into the renal parenchyma, renal pelvis or periureteral tissue) and/or N+ (lymph node positive) at final pathology.
Both the cox and logistic multivariate regression analyses were performed in a backward manner. To pre-select included candidate variables a cut off of p<.2 in univariate analyses were chosen. For all analyses, a p value less than .05 were considered statistically significant. All analyses were performed by use of SPSS version 26.0 (IBM, Armonk, NY).
Results
The patient demographics and tumour characteristics are presented in .
Table 1. Patient and tumour characteristics.
Prediction of survival
The 5- and 10 year CSS of the whole cohort was 79% and 75%, respectively. The 5- and 10-year OS was 60% and 35%, respectively. Patients with OCD had a higher CSS (93% vs. 55%, p<.001) and OS (71% vs. 42%, p<.001) compared to patients with NOCD (Supplementary Figures 1 and 2). Mean (median) follow-up time in patients alive without recurrence was 58 (47) months. In a multivariate cox regression analysis, pathological tumour stage and ECOG were significant predictors of all-cause mortality. Pathological tumour stage predicted UTUC specific mortality (Supplementary Tables 1 and 2).
The presence of local invasion (64% vs. 86%, p = 0.002), pathological lymph nodes (41% vs. 83%, p<.001) and fatty tissue reaction surrounding the tumour (58% vs. 84%, p=.001) at CT predicted CSS in the present material. Regarding patient factors, ECOG 0 predicted improved CSS compared to ECOG ≥ 1 (85% vs. 70%, p=.03). No other features regarding the patient, radiological examinations or ureterrenoscopic variables predicted CSS in this study.
Prediction of tumour stage
Patient features
The results of the univariate regression analyses regarding patient factors ability to predict NOCD are shown in . No patient factors were shown to be predictors of NOCD at final pathology in this study.
Table 2. Univariate odds ratios for non-organ confined disease according to patient risk variable.
Radiological variables
The results of the univariate regression analyses regarding CT variables are shown in . Non-organ confined tumours were larger than organ-confined tumours (4.8 cm vs. 3.9 cm, p=.006). The presence of reactive oedema in the fatty tissue surrounding the tumour predicted NOCD (OR 2.55, p=.016). This was particularly true for tumours in the ureter with an OR of 8.0 for those tumours (p=.002). The presence of pathological lymph nodes and local invasion on CT predicted NOCD (OR 14.5, p=.001 and 5.31, p<.001, respectively). The sensitivity and specificity of local invasion at CT to predict NOCD was 49% and 85%, respectively. The sensitivity and specificity of pathological lymph nodes CT to predict NOCD was 22% and 98%, respectively. Hydronephrosis was present in 114 of 173 patients (66%). Contrast enhancement and the presence of hydronephrosis did not predict NOCD in this patient material.
Ureterrenoscopic variables
The results of the univariate regression analyses regarding the ureterrenoscopic variables are described in . A diagnostic URS with biopsy was performed in 95 patients. In 66 (69%) of the patients, the biopsy could be used to confirm UTUC diagnosis, and in 57 (60%) the biopsy material was sufficient to determine tumour grade. Presence of high-grade tumour at biopsy predicted NOCD (OR 3.59, p=.04). The sensitivity and specificity of high-grade biopsy to predict NOCD was 63% and 68%, respectively. Out of 34 low-grade tumours at biopsy, 12 (35%) were upgraded to high-grade at final pathology after RNU. No high-grade tumours were downgraded. The tumour stage at biopsy did not predict NOCD in the present material. A cytology sample was taken in 60 patients, 43 of these taken during URS. Malignant cells were detected among 37 (62%) of these. Malignant cells at cytology did not predict NOCD in the present material.
Table 4. Results of the analyses made from the ureterenoscopic variables regarding determination of biopsy tumour grade and prediction of non-organ confined disease at final pathology after nephroureterectomy.
Multivariate analyses
All variables with a predictive value for NOCD with a p-value <0.2 (smoking status, presence of hydronephrosis, fatty tissue reactive oedema or pathological lymph nodes together with tumour size and local invasion at CT) were entered into a multivariate logistic regression analysis. The results from these analyses are shown in . Pathological lymph nodes and local invasion on CT remained significant predictors of NOCD (6.21, p=.03 and 3.36, p=.004, respectively).
Table 5. Multivariate regression analyses for prediction of NOCD at final specimen according to risk variables.
Discussion
In this study, several variables from the CT images and biopsy tumour grade were identified as preoperative predictors for NOCD. With the exception of biopsy tumour grade and tumour size, these factors also predicted survival.
One of the unanswered questions regarding the preoperative diagnostic procedures before RNU for UTUC is the role of the diagnostic URS. In the current EAU-guidelines, a URS is recommended if imaging and cytology are not sufficient for the diagnosis and/or risk-stratification of the tumour [Citation8]. There is a role for the diagnostic URS in case the result of the CT is unclear and further examinations to set the correct diagnosis are necessary. Moreover, another indication is if the patient is a potential candidate for nephron-sparing treatment, such as a segmental ureter resection or endoscopic laser tumour ablation. The evaluation of the diagnostic URS in these settings was not among the aims of this paper, and will not be discussed further here.
When it comes to the staging of UTUC, the role of the diagnostic URS is much more unclear. Tumour grade is regarded as a predictor of tumour stage at final pathology [Citation2]. However, the problem with tumour grade from biopsy is that it is often not possible to get a biopsy at all at the procedure, and in case a low-grade biopsy is found, it is frequently upgraded to high-grade at final pathology. In the present paper, the biopsy could confirm UTUC diagnosis only in 69% of the cases. This might seem like a low rate of histological verification, but is in line with the findings in a prospective study evaluating URS biopsies. Breda et al. found that a histological evaluation was possible in 78% of the biopsies, with complete histopathological assessment only among 46%. [Citation11]. One could of course turn this around and say that histological confirmation from URS biopsy is possible in a majority of the cases, and such verification is a requirement for the oncologists before considering neo-adjuvant chemotherapy. However, histological verification can also be achieved through cytology at cystoscopy which is mandatory before RNU. In the present cohort, the sensitivity of cytology to verify UTUC was 62%, not very different from the sensitivity of 69% from the URS biopsy. Another aspect of the URS biopsy was that 35% of the low-grade biopsies were upgraded to high-grade at final pathology. Such upgrading is a known phenomenon. A meta-analysis on the topic analysing more than 2000 URS biopsies from 23 studies concluded that the pooled upgrading rate from low- to high-grade tumours was at 34% [Citation12], and thus in line with our results.
In spite of the demonstrated limitations regarding the diagnostic URS, biopsy tumour grade was still a significant predictor of NOCD at univariate analyses in the present material, and further analyses were made to assess potential clinical benefit. The sensitivity and specificity of high-grade tumour at biopsy to predict NOCD were 63% and 68%, respectively. We think that the accuracies of these predictions are too low to be clinically useful. Furthermore, there is also the aspect that URS requires time and resources, and thus delays definite treatment. Finally, in two meta-analyses, an increased risk of post-RNU bladder recurrence has been demonstrated among patients examined with URS [Citation13,Citation14].
To conclude, URS as a diagnostic measure among patients where a decision for RNU has already been made has considerable limitations, and will only rarely influence the decision about intensified treatment. It causes curative treatment delay and an increased risk of bladder recurrence after RNU. We argue that a preoperative URS should be spared for cases where the diagnosis is uncertain or when nephron-sparing treatment might be an option.
How can our findings be used in a clinical practice? If a URS is omitted in the preoperative diagnostic work-up, the clinician is left with the findings (a) in the CT scan and (b) at cystoscopy visit when deciding on potential intensified treatment. There is emerging evidence of the efficacy of perioperative chemotherapy, but selecting the appropriate patients for this is challenging. Indeed, in Norway, different approaches to perioperative chemotherapy at different hospitals exist. The POUT study has recently demonstrated the efficacy of adjuvant chemotherapy in case of muscle-invasive disease [Citation4], and one could argue that the best strategy is simply to wait for final pathology and then decide whether to give adjuvant chemotherapy or not. However, neo-adjuvant chemotherapy in the treatment of UTUC has some appealing advantages. Neo-adjuvant chemotherapy before cystectomy for bladder cancer has demonstrated survival benefit, and is standard treatment according to guidelines [Citation15]. Second and perhaps more importantly, a RNU will inevitably reduce the kidney function of the patient. We know that this will make a significant proportion of the patients ineligible for adjuvant chemotherapy due to reduced kidney function postoperatively. On the other hand, giving neo-adjuvant to all chemo-eligible patients undergoing RNU will inevitably result in giving a toxic and potentially lethal treatment to a large group of patients with non-muscle invasive disease. In the present material, the proportion of patients with Ta and T1 disease was 47%. The proportion of patients with organ-confined disease was 62%. We think that giving neo-adjuvant treatment to patients with non-muscle invasive disease would result in unacceptable side effects to a patient group where the potential benefit of the treatment is highly debatable.
So how can we select the appropriate patients for neo-adjuvant chemotherapy? Both pathological lymph nodes and fatty tissue oedema predicted NOCD in the present cohort. However pathological lymph nodes had a very low sensitivity of 22% in predicting NOCD and would result in missing out many potential candidates for neo-adjuvant chemotherapy. Fatty tissue reaction surrounding the tumour also demonstrated predictive ability, but this is a feature that to our knowledge has not been demonstrated as a predictor for tumour stage after RNU before. Its predictive abilities should be confirmed in further studies before it is taken into standard clinical practice.
The presence of local invasion on CT seems a more promising feature to use in patient selection for neo-adjuvant chemotherapy. It was found to be a significant predictor of NOCD and survival in the present cohort. The predictive ability of local invasion at CT has been described by other authors. In a diagnostic model presented by Favaretto et al., local invasion at CT was found to predict NOCD and was used as a part of their presented diagnostic model [Citation16]. A recently published study reported a sensitivity and specificity of 75% and 83% correspondingly using CT to detect advanced stage (T3/T4) UTUC [Citation17].
Local invasion on CT in this study showed a relatively low sensitivity of 48% but a corresponding high specificity of 85% in predicting NOCD. A sensitivity of 48% might seem unacceptably low, but this sensitivity as a cut off is the same as suggested in a recently published model by Petros et al. The authors of that study generated a predictive model that included findings at CT, URS and blood samples to reach a sensitivity of 49% and specificity of 95% in predicting NOCD [Citation18]. The higher specificity demonstrated in their model is beneficiary, but comes at the cost of a highly complicated model using parameters from URS and blood samples in addition to findings at the CT in a nomogram. In our opinion, the complexity of the model makes it less useful in day to day clinical practice.
By using the accuracy from this study, invasion at CT as a guide for who could benefit from neo-adjuvant, would result in only half of the patients with NOCD receiving chemo as a neoadjuvant. However, few patients with non-muscle invasive disease would be ‘overtreated’ with chemotherapy. The patients with muscle-invasive disease that did not receive neo-adjuvant chemotherapy could be good candidates for adjuvant chemotherapy. If the kidney function was still acceptable (GFR > 55), Cisplatin-based regimens would be preferred. If not, Carboplatin based regimens could be an option. Both these regimens of adjuvant chemotherapy were included and shown to be beneficial in the POUT study. High-quality randomized studies comparing neo-adjuvant with adjuvant chemotherapy in the treatment of UTUC are urgently needed. Pending evidence from such high-quality studies, we suggest that this very simple and readily available feature can be used as a guide for selecting patients for either neo-adjuvant or adjuvant chemotherapy. Its use is demonstrated in a proposed flowchart ().
Figure 1. Demonstrating a proposed flowchart that can be used in the selection of patients for perioperative chemotherapy and/or extended LND in adjunct to radical nephroureterectomy for upper tract urothelial carcinoma. UTUC: upper tract urothelial carcinoma. RNU: Radical nephroureterectomy. LND: Lymph node dissection; NOCD: non-organ confined disease; OCD: organ-confined disease; MID: muscle-invasive disease; GFR: glomerular filtration rate.
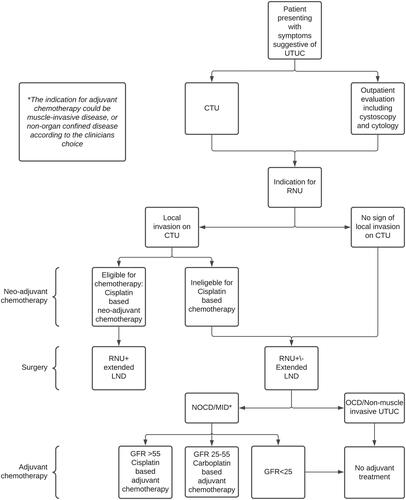
The same approach can be used when selecting patients for extended LND. Current EAU guidelines recommend that a template-based LND should be performed during RNU [Citation8]. In case pathological but resectable lymph nodes are present on the preoperative CT, an LND should be performed. However, the presence of lymph nodes metastasis is strongly dependent on tumour stage, and an LND appears to be unnecessary in Ta/T1 tumours [Citation19]. In case the CT is negative for pathological lymph nodes but local invasion is present, an LND could be performed with an indication similar to that of neo-adjuvant chemotherapy ().
The inclusion of survival analyses presented in this study serve two purposes. First, the 5-year DSS of 79% in this study is comparable to the DSS presented in larger cohorts [Citation20,Citation21]. This suggests that our cohort is a representative sample of UTUC cohorts in general, and could increase the generalizability of our findings. Second, the same predictors of tumour stage at CT also predicted survival in our study. This was as expected, but these findings further underscore the importance of the predictors we discovered both regarding stage and survival.
Strengths and weaknesses
The strengths of this article include using patients from two larger centres in Norway to allow enough patients to make robust analyses of staging and survival. However, increasing the number of patients by collaborating with additional centres would have increased the generalizability of our findings further. All CT scans were re-evaluated by a uro-radiologist and all re-evaluations of histopathological specimens were performed by uro-pathologists to increase data quality as much as possible. The weakness of the study is its retrospective study design, with the inherent weaknesses associated with this study design.
Conclusion
Several features in the preoperative diagnostics before RNU for UTUC were shown to predict tumour stage and survival in this study. Of these, local invasion on CT seems to be the most promising feature when selecting the appropriate patients for intensified treatment for UTUC. The role of the diagnostic URS in the staging of UTUC seems limited. The preoperative staging of UTUC before RNU remains challenging, and further studies on the topic are warranted.
Supplemental Material
Download Zip (460.2 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–1233.
- Abouassaly R, Alibhai SM, Shah N, et al. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology. 2010;76(4):895–901.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268–1277.
- Matin SF, Margulis V, Kamat A, et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer. 2010;116(13):3127–3134.
- Inokuchi J, Kuroiwa K, Kakehi Y, et al. Role of lymph node dissection during radical nephroureterectomy for upper urinary tract urothelial cancer: multi-institutional large retrospective study JCOG1110A. World J Urol. 2017;35(11):1737–1744.
- Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncol. 2017;35(8):852–860.
- Rouprêt M, Babjuk M, Comperat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111–122.
- Eble JN, Sauter G, Epstein JI. Pathology & genetics of tumors of the urinary system and male genital organs. Lyon (France): IARC Press; 2004.
- Brierly JG, Wittekind C. TNM classification of malignant tumors. 8th ed. Oxford (UK): Wiley Blackwell; 2017.
- Breda A, Territo A, Sanguedolce F, et al. Comparison of biopsy devices in upper tract urothelial carcinoma. World J Urol. 2019;37(9):1899–1905.
- Subiela JD, Territo A, Mercadé A, et al. Diagnostic accuracy of ureteroscopic biopsy in predicting stage and grade at final pathology in upper tract urothelial carcinoma: systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:1989–1997.
- Marchioni M, Primiceri G, Cindolo L, et al. Impact of diagnostic ureteroscopy on intravesical recurrence in patients undergoing radical nephroureterectomy for upper tract urothelial cancer: a systematic review and meta-analysis. BJU Int. 2017;120(3):313–319. Sep
- Guo RQ, Hong P, Xiong GY, et al. Impact of ureteroscopy before radical nephroureterectomy for upper tract urothelial carcinomas on oncological outcomes: a meta-analysis. BJU Int. 2018;121(2):184–193.
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475.
- Favaretto RL, Shariat SF, Savage C, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int. 2012;109(1):77–82. Jan
- Yu SH, Hur YH, Hwang EC, et al. Does multidetector computed tomographic urography (MDCTU) T staging classification correspond with pathologic T staging in upper tract urothelial carcinoma? Int Urol Nephrol. 2021;53(1):69–75.
- Petros FG, Qiao W, Singla N, et al. Preoperative multiplex nomogram for prediction of high-risk nonorgan-confined upper-tract urothelial carcinoma. Urol Oncol. 2019;37(4):292.e1–292–e9.
- Lughezzani G, Jeldres C, Isbarn H, et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology. 2010;75(1):118–124.
- Rouprêt M, Hupertan V, Seisen T, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. 2013;189(5):1662–1669. May
- Lughezzani G, Jeldres C, Isbarn H, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer. 2009;45(18):3291–3297.
