Abstract
Objective
To assess the value of second-look resection (SLR) in stage T1 bladder cancer (BCa) with respect to progression-free survival (PFS), and also the secondary outcomes recurrence-free survival (RFS), bladder-cancer-specific survival (CSS), and cystectomy-free survival (CFS).
Patients and methods
The study included 2456 patients diagnosed with stage T1 BCa 2004–2009 with 5-yr follow-up registration in the nationwide Bladder Cancer Data Base Sweden (BladderBaSe). PFS, RFS, CSS, and CFS were evaluated in stage T1 BCa patients with or without routine SLR, using univariate and multivariable Cox regression with adjustment for multiple confounders (age, gender, tumour grade, intravesical treatment, hospital volume, comorbidity, and educational level).
Results
SLR was performed in 642 (26%) individuals, and more frequently on patients who were aged < 75 yr, had grade 3 tumours, and had less comorbidity. There was no association between SLR and PFS (hazard ratio [HR] 1.1, confidence interval [CI] 0.85–1.3), RFS (HR 1.0, CI 0.90–1.2), CFS (HR 1.2, CI 0.95–1.5) or CSS (HR 1.1, CI 0.89–1.4).
Conclusions
We found similar survival outcomes in patients with and patients without SLR, but our study is likely affected by selection mechanisms. A randomised study defining the role of SLR in stage T1 BCa would be highly relevant to guide current praxis.
1. Introduction
The diagnosis and treatment of stage T1 bladder cancer (BCa) relies on a complete initial transurethral resection of the bladder tumour (TURB). Even after a complete primary resection, current guidelines unanimously recommend that a second-look resection (SLR) be carried out within 2–6 wk of the primary procedure, although there is only one randomized trial supporting such practice (level of evidence 1B or 2) [Citation1]. The rationale for performing SLR is to ensure a complete resection of the tumour. That strategy is supported by findings of residual tumour in up to 71% of patients at SLR [Citation1], and it enables biopsies to be obtained from the prostatic urethra if indicated, as well as from abnormal-looking urothelium to rule out concomitant carcinoma in situ (if not performed during the primary resection) [Citation2]. To date, only one randomised study has evaluated SLR in stage T1 BCa, and the results showed improvement in both progression-free survival (PFS) and recurrence-free survival (RFS) [Citation3], although the adjuvant therapy used in that investigation was mitomycin and not bacillus Calmette-Guérin (BCG), which is the current standard. On the other hand, the effect-size for the SLR-intervention in that study on both RFS and PFS is larger than the expected differences for those outcome measures between adjuvant instillations with mitomycin and BCG [Citation4]. The remaining evidence for SLR emanates from retrospective data hampered by studying heterogeneous cohorts, being influenced by selection bias, and also showing conflicting results [Citation5–10]. However, some of the series included in that evaluation did not use adjuvant BCG instillations, or even no adjuvant instillations at all, and the largest study assessed did not report progression-free survival, the most important outcome measure in stage T1 BCa. We performed the present ’real life’ study to evaluate the potential benefit of routine SLR in a population-based setting with a 5-yr follow-up, investigating PFS. In addition, we analysed RFS, cancer-specific survival (CSS), and cystectomy-free survival (CFS).
2. Patients and methods
2.1. Study population
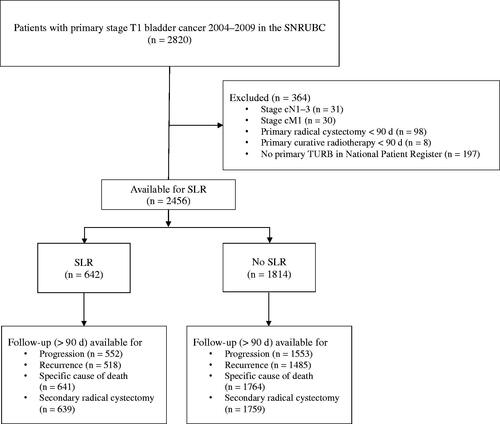
A population-based cohort of 2820 patients diagnosed with stage T1 primary BCa during the period 2004–2009 was identified in Bladder Cancer Data Base Sweden (BladderBaSe) (those years were chosen being the last five years with available data from a 5-year follow-up form on recurrence and progression, see below), which covers all patients included in the Swedish National Registry of Urinary Bladder Cancer (SNRUBC) with linked data from national registers and demographic data [Citation11]. We excluded patients treated with primary radical cystectomy or primary external radiotherapy, patients with primary lymph node positive or metastatic disease, and patients without a primary TURB filed in the National Patient Register (–28 d to +90 d from registered date of diagnosis) (i.e. for example in a patient with severe comorbidity when only a biopsy under local anesthesia was obtained) (). Thus, 2456 patients remained for analyses. To minimise the possibility of SLR having been performed for a clinical recurrence, we defined SLR as a second TURB conducted 28–56 days after the initial procedure reported in the National Patient Register (in- and/or out-patient registries ICD-10 code KCD02) ().
2.2. Measures
Tumour grade was categorised according to WHO 1999, but no systematic pathological re-evaluation of grade or stage were undertaken in the study cohort. Progression (defined as (but not indicated separately) muscle-invasive local recurrence or nodal and/or distant metastasis) and recurrence were ascertained from the SNRUBC 5-year follow-up form distributed 5 yr after diagnosis. To identify radical cystectomies, we retrieved data from the National Patient Register (ICD-10 code KCC). Date and cause of death were obtained from the Cause of Death Register.
Charlson Comorbidity Index (CCI) was calculated based on a list of diseases, with a specific weight assigned to each disease category according to data from the National Patient Register. The separate weights were collated to give an overall score, categorising morbidity as follows: 0 = none, 1 = mild, 2 = intermediate, and ≥3 = severe. Educational level was retrieved from Statistics Sweden and categorised as low (≤9 y of education), intermediate (10–12 y), and high (≥13 y), corresponding to mandatory school, high school, and college or university [Citation11]. Information about smoking history and status was not available.
Hospital volume was calculated as number of registered primary T1 BCa operations (ICD-10 code KCD02) the year of diagnosis for the patient treated at the hospital in question. Thereafter, from 73 reporting hospitals, the median number of operations for all units and years was calculated and used as cut-off for a dichotomised variable of hospital volume (four or more annual operations). Age was stratified above or below the mean age in the study population (≥75 and <75 yr).
Information on adjuvant intravesical treatment in the SNRUBC is limited to type of treatment (BCG or chemotherapy), thus no data is available beyond an induction course such as number of instillations administered.
2.3. Statistical analyses
Univariable and multivariable logistic regression models were applied to analyse the association between potential factors affecting the use of SLR. The primary analysis was a log-rank test comparing progression-free (PFS) survival in patients who did and those who did not undergo SLR. In addition, we evaluated recurrence-free (RFS), cancer-specific (CSS) and cystectomy-free survival (CFS) (cystectomy beyond 90 days of bladder cancer diagnosis during the follow-up period). The Kaplan–Meier method was used to visualise survival, and Cox univariable and multivariable regression models were applied to adjust for confounders. All survival analyses were calculated from date of primary TURB. For CSS and CFS, 31 December 2014 was regarded as the end of follow-up, and patients were censored at date of death, emigration, or end of follow-up, whichever occurred first. However, when calculating RFS and PFS, follow-up date according to the 5-yr follow-up form was the end of follow-up.
In a sensitivity analysis, to account for the heterogeneous study population and to mimic a clinically relevant ‘trial population’ for a randomised study, we also performed the above-mentioned survival analyses in the subgroup of patients with tumour grade 2 or 3, all of whom had been given adjuvant intravesical BCG instillations. Similarly, due to the finding of a low proportion of patients subjected to SLR in the Western Health Care Region, possibly implicating different criteria for selecting patients for SLR, we performed separate sensitivity analyses excluding this region.
After finding statistically significant association of patients’ age and tumour grade and use of SLR, suggesting that clinicians were more likely to perform SLR based on these aspects, we also performed separate survival analyses on the subgroup of patients aged < 75 yr and the subgroup with primary tumour grade 3. Also, due to the low proportion of SLR and a significant proportion of second TURB performed in the time interval 57–90 days after the first procedure, supplemental survival analyses were performed using a higher upper limit (within 90 d after the initial TURB-procedure) when defining a SLR. Furthermore, to investigate the potential effect of time to SLR, we evaluated the primary outcome measure (PFS) in subgroups based on time to SLR: 28–42, 43–56, 57–70, or 71–90 d. All statistical analyses were performed with the R statistical package [Citation12].
2.4. Ethical review
The study was approved by the Ethics Committee at Uppsala University, Uppsala, Sweden (EPN 2015/277).
3. Results
Background characteristics for all study participants are presented in . Considering all 2456 patients included in the study, mean age at the primary TURB was 75 (standard deviation [SD] 11) yr, and 23% were women. Median (IQR) time to SLR was 45 (40-50) days with the following distribution: 28-42 d (42%), 43-56 d (58%). Mean follow-up time was 4.4 (SD 2.2) yr for PFS, 3.3 (SD 2.5) yr for RFS, 5.3 (SD 3.1) yr for CFS, and 5.5 (SD 3.0) yr for CSS.
Table 1. Descriptive data.
It was more likely for SLR to be performed on patients aged < 75 yr at diagnosis, those with grade 3 tumours, and those who received subsequent adjuvant intravesical treatment. In contrast, it was less likely for SLR to be done on patients with comorbid conditions and those treated in the Western Health Care Region in Sweden ().
Table 2. Binary logistic regression of factors associated with SLR in patients with primary stage T1 bladder cancer (BCa) (likelihood of SLR in categories of covariates).
Visualisation of survival estimates (PFS, RFS, CFS and CCS) for the total study population is presented in .
Figure 2. Kaplan–Meier plots for progression-free survival (PFS), recurrence-free survival (RFS), bladder-cancer-specific survival (CSS), and cystectomy-free survival (CFS).
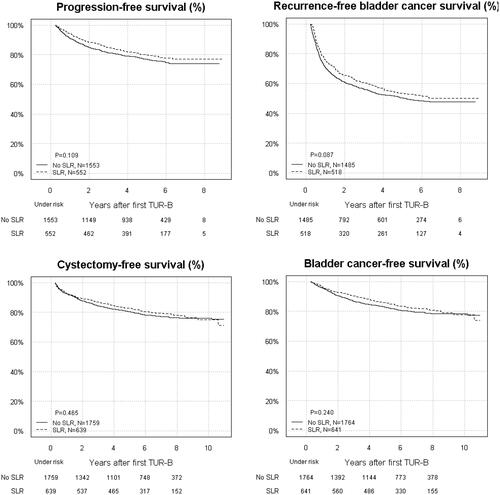
In general, PFS was similar in patients subjected to SLR (hazard ratio [HR] 1.1, confidence interval [CI] 0.85–1.3) () and the no-SLR group. For RFS, the HRs were close to unity (). Similarly, CSS and CFS estimates were similar for SLR vs. no-SLR ().
Table 3. Hazard ratios (HRs) with 95% confidence intervals (CIs) for progression-free survival (PFS) and recurrence-free survival (RFS) assessed with cox regression in stage T1 bladder cancer treated with or without second look resection (SLR), including stratified analyses in the subgroups of patients aged <75 yr and with grade 3 tumours, excluding patients treated in the Western Health Care Region and in a tentative trial population (grade 2 + 3 tumours treated with BCG).
Table 4. Hazard ratios (HRs) with 95% confidence intervals (CIs) for cancer-specific survival (CSS) and cystectomy-free survival (CFS) assessed with Cox regression in stage T1 bladder cancer patients treated with or without second-look resection (SLR), including stratified analyses in the subgroups of patients aged <75 yr with grade 3 tumours, excluding patients treated in the Western Health Care Region and in a tentative trial population (grade 2 + 3 tumours treated with BCG).
Among the individuals in the ‘trial population’ who were deemed to be eligible to participate in a randomised trial (i.e. had grade 2 and 3 tumours treated with adjuvant BCG instillations), we noted similar survival outcomes (PFS, RFS, CSS, or CFS) for the SLR and the no-SLR group. Likewise, no change in any of the survival outcomes were apparent when the Western Health Care Region was excluded ( and ).
Supplementary survival analyses using a wider definition of SLR (with upper limit of 90 days after initial TURB to define SLR) identified 1102 patients with SLR corresponding to 45% of the study population, with the following distribution 28–42 d (24%), 43–56 d (36%), 58–72 d (24%) and 73–90 d (16%). This resulted in similar estimates for all survival outcomes, apart from an increased risk of cystectomy during follow-up for those subjected to a SLR (HR 1.3, CI 1.0–1.5) (Supplemental Table 1 and Supplemental Table 2). Moreover, no association between PFS and time to SLR were detected when time to SLR was stratified in four groups according to number of days from the primary resection (28–42, 43–56, 57–70, or 71–90 d) (data not shown).
4. Discussion
This large population-based study conducted in Sweden showed that only 26% of stage T1 patients received a SLR within 8 weeks and similar survival outcomes in patients who were or were not treated with SLR. We identified associations between performing SLR and patient age, tumour grade 3, intravesical treatment, and comorbidity.
The main limitation of this study in addition to the retrospective observational study design, is the lack of more detailed information on tumour characteristics, such as residual tumour at SLR (i.e. a measure of the completeness of the primary TURB) [Citation13], depth of lamina propria invasion [Citation14], and presence of lymphovascular invasion [Citation15] or variant histologies. All these factors are known to independently affect risk of progression and their presence or absence might have influenced the choice to perform SLR. Likewise, another limitation is a lack of information about some clinical factors associated with PFS that also could imply selection for SLR, such as tumour size, number of tumours, and presence of carcinoma in situ. Furthermore, there was no information regarding whether muscle was or was not present in the primary specimen [Citation5], a factor that has also been reported to affect PFS. These drawbacks may have driven the results towards unity and led to underestimation of the benefit of SLR. Moreover, there was no information about number of BCG courses administered. Another weakness of this study is that muscle-invasive disease was detected at SLR in an unknown number of patients, and hence those patients were registered as having clinically muscle-invasive disease and not stage T1 disease. This would have disfavoured the non-SLR group in that the corresponding patients were not removed and thus possibly partly compensated for the selection of patients with more aggressive disease to SLR. On the other hand, a strength of our investigation is the large nationwide study population with high coverage in the SNRUBC [Citation11] and different practice patterns with proportions of SLR performed between health care regions ranging from 20% to 53% (). Also, linked data from high-quality registries enabled adjustment for other confounders such as comorbidity and educational level.
Today, SLR is recommended for stage T1 disease in all guidelines, and this advice is based on the following: existing observational data suggesting frequent residual tumour at SLR (65% and 71%, respectively) [Citation1,Citation16]; a randomised study using suboptimal adjuvant treatment (mitomycin instead of BCG) and excluding patients with carcinoma in situ that showed worse PFS (HR 3.5) without SLR [Citation17]; and a large recent systematic review concluding that SLR ‘may improve outcomes for cancers initially staged as T1’ [Citation1]. However, both the cited systematic review [Citation1] and another recent systematic review and meta-analysis [Citation18] proposed that a prospective randomised study be performed to determine the value of SLR and assess PFS in putative subgroups of stage T1 disease. Such a proposal is also supported by our finding of similar survival outcomes with or without SLR in the stratified analyses of patients aged < 75 yr and those with grade 3 tumours, i.e. the patients that clinicians were actually more prone to select for SLR. Furthermore, in our study, a sensitivity analysis in the subgroup of patients suitable for being included in such a trial (i.e. those with grade 2 and 3 tumours also receiving adjuvant BCG instillations) showed no effect of SLR on survival in this ‘trial population’. Nevertheless, our analyses might have underestimated the effect of SLR and similar outcomes in the assessed groups indicate that the patient group subjected to a SLR is associated with worse prognosis and that the SLR per se could be pushing these estimates towards unity.
Delayed time to SLR has been proposed to decrease RFS and PFS, if the SLR is performed 6 or 8 wk, respectively, after the primary resection [Citation7,Citation19]. However, as opposed to previous reports, the present study showed no association with time to SLR. In our experience, the health care situation in Sweden often leads to delayed surgery, from the years 2015 to 2017 median (IQR) time to SLR was 42 (33-60) days despite standardized care pathways with defined shorter lead times [Citation20]. In supplemental analyses, we used a wider time frame for SLR (i.e. 28–90 d) when exploring the effect of time to SLR on survival, because plotting of the timing of secondary resections revealed that a significant proportion had been performed during the period 6–12 wk after the primary procedure. With this definition, SLR proportion increased to 45%, yet the main results and conclusions remained apart from an increased risk of cystectomy during follow-up for those subjected to a SLR (HR 1.3, CI 1.0–1.5). This suggests confounding by selection of patients with more severe stage T1 disease for SLR.
Thus, unmet needs today include being able to spare some patients (those without remaining tumour) an unnecessary SLR and being able to avoid a second fragmentation of an invasive cancer tumour at SLR in some subjects by a priori defining indications for primary cystectomy based on prognostic information at the first resection. Magnetic resonance imaging is a modality that has been suggested to predict PFS in stage T1 bladder cancer that could be a future option to select patients to primary cystectomy [Citation21]. Indeed, primary cystectomy can also be proposed without performing a SLR based on the presence of variant histologies, but also on the finding of deep lamina propria invasion and lymphovascular invasion, which represent the two factors observed to have the relatively most pronounced effect on risk of progression in the hitherto largest meta-analysis of high-grade T1 disease [Citation22]. It has been suggested that, when designing a prospective randomised study, absence of detrusor muscle in the first TURB specimen could be a tentative indication to perform SLR, a conclusion based on survival advantages found in patients subjected to SLR only if no muscle was present in the primary specimen [Citation5]. However, limiting inclusion to patients with absence of detrusor in the TURB specimen in such an investigation would probably not suffice as an enrolment criterion, because in only 6% of patients does a high-quality primary resection result in T1 tumours lacking detrusor in the specimen [Citation23]. Instead, to further define the population that might not require SLR in stage T1 disease, we propose a prospective randomised study with non-inferiority design that includes stratification of both patient- and tumour-related factors. This would shorten the time to starting BCG-instillations, where delayed adjuvant instillations have been reported to decrease survival [Citation24]. Still, further definition and selection of the patients that benefit from early cystectomy is of highest importance in future workup of stage T1 disease.
5. Conclusions
The proportion of patients with stage T1 disease that received a SLR was low (26%) in this population-based nationwide study. Survival outcomes in patients treated with SLR and those without SLR were similar, yet our analyses are likely influenced by selection mechanisms and limited by the lack of information of e.g. completeness of the primary TURB. Consequently, given a priori criteria for primary cystectomy based on information obtained at the first TURB, a prospective investigation aimed at defining when to refrain from SLR in the remaining patients is needed to improve the treatment of stage T1 BCa.
Supplemental Material
Download MS Word (23.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2018;73(6):925–933.
- https://uroweb.org/individual-guidelines/oncology-guidelines/.
- Eroglu A, Ekin RG, Koc G, et al. The prognostic value of routine second transurethral resection in patients with newly diagnosed stage pT1 non-muscle-invasive bladder cancer: results from randomized 10-year extension trial. Int J Clin Oncol. 2020;25(4):698–704.
- Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette–Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247–256.
- Gontero P, Sylvester R, Pisano F, et al. The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette–Guérin. BJU Int. 2016;118(1):44–52.
- Angulo JC, Palou J, Garcia-Tello A, et al. Second transurethral resection and prognosis of high-grade non-muscle invasive bladder cancer in patients not receiving bacillus Calmette–Guerin. Actas Urol Esp. 2014;38(3):164–171.
- Krajewski W, Zdrojowy R, Dembowski J, et al. The optimal timing of restaging resection before introduction of bacillus Calmette–Guerin immunotherapy in patients with high-risk non-muscle-invasive bladder cancer. Urol Int. 2019;102(1):60–68.
- Grimm MO, Steinhoff C, Simon X, et al. Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study. J Urol. 2003;170(2 Pt 1):433–437.
- Calo B, Chirico M, Fortunato F, et al. Is repeat transurethral resection always needed in high-grade T1 bladder cancer? Front Oncol. 2019;9:465.
- Tseng WH, Liu CL, Huang SK, et al. Therapeutic benefit of second-look transurethral resection of bladder tumors for newly diagnosed T1 bladder cancer: a single-center experience. Int Urol Nephrol. 2019;51(8):1335–1342.
- Haggstrom C, Liedberg F, Hagberg O, et al. Cohort profile: The Swedish National Register of Urinary Bladder Cancer (SNRUBC) and the Bladder Cancer Data Base Sweden (BladderBaSe). BMJ Open. 2017;7(9):e016606.
- R Core team 2020. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. Available from: https://www.R-project.org/.
- Ferro M, Vartolomei MD, Cantiello F, et al. High-grade T1 on re-transurethral resection after initial high-grade T1 confers worse oncological outcomes: results of a multi-institutional study. Urol Int. 2018;101(1):7–15.
- Fransen van de Putte EE, Otto W, Hartmann A, et al. Metric substage according to micro and extensive lamina propria invasion improves prognostics in T1 bladder cancer. Urol Oncol. 2018;36(8):361.e7–361.e13.
- Andius P, Johansson SL, Holmang S. Prognostic factors in stage T1 bladder cancer: tumor pattern (solid or papillary) and vascular invasion more important than depth of invasion. Urology. 2007;70(4):758–762.
- Patschan O, Holmang S, Hosseini A, et al. Second-look resection for primary stage T1 bladder cancer: a population-based study. Scand J Urol. 2017;51(4):301–307.
- Divrik RT, Sahin AF, Yildirim U, et al. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol. 2010;58(2):185–190.
- Krajewski W, Nowak L, Poletajew S, et al. The impact of restaging transurethral resection of bladder tumour on survival parameters in T1 non-muscle invasive bladder cancer – systematic review and meta-analysis. J Endourol. 2020;34(8):795–804.
- Baltaci S, Bozlu M, Yildirim A, et al. Significance of the interval between first and second transurethral resection on recurrence and progression rates in patients with high-risk non-muscle-invasive bladder cancer treated with maintenance intravesical Bacillus Calmette–Guerin. BJU Int. 2015;116(5):721–726.
- Nilbert M, Blackberg M, Ceberg J, et al. Diagnostic pathway efficacy for urinary tract cancer: population-based outcome of standardized evaluation for macroscopic haematuria. Scand J Urol. 2018;52(4):237–243.
- Yajima S, Yoshida S, Takahara T, et al. Usefulness of the inchworm sign on DWI for predicting pT1 bladder cancer progression. Eur Radiol. 2019;29(7):3881–3888.
- Martin-Doyle W, Leow JJ, Orsola A, et al. Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol. 2015;33(6):643–650.
- Sorenby A, Baseckas G, Bendahl PO, et al. Reducing recurrence in non-muscle-invasive bladder cancer by systematically implementing guideline-based recommendations: effect of a prospective intervention in primary bladder cancer patients. Scand J Urol. 2019;53(2-3):109–115.
- Krajewski W, Moschini M, Chorbinska J, et al. Delaying BCG immunotherapy onset after transurethral resection of non-muscle-invasive bladder cancer is associated with adverse survival outcomes. World J Urol. 2020. doi: 10.1007/s00345-020-03522-3.