Abstract
Purpose
End-stage renal disease (ESRD) is a known risk factor for the development of renal cell carcinoma (RCC). This case-control study was performed to assess the risk in a nationwide cohort and evaluate tumor characteristics and survival in the ESRD-RCC population.
Methods
In this study, 9,299 patients with RCC identified in the National Swedish Kidney Cancer Register from 2005 until 2014 and 93,895 matched controls were linked to the Swedish Renal Registry and the National Patient Register. ESRD was defined as chronic kidney disease stage 5, kidney transplantation or kidney dialysis 0–40 years before the diagnosis of RCC.
Results
A total of 117 patients with ESRD and subsequent RCC were identified and compared with 9,087 patients with RCC. There was a 4.5-times increased risk for RCC among ESRD patients (95% CI = 3.6–5.6; p < 0.001) compared to matched controls. Longer time with ESRD increased the risk of RCC (ESRD > 9 years, OR = 10.2, 95% CI = 7.0–14.8). The ESRD-RCC patients were younger (p = 0.002), had smaller tumors (p < 0.001) and had lower tumor stage (p = 0.045). The incidence of papillary and chromophobe RCC was higher and clear cell RCC lower among the ESRD patients (p < 0.001). The 5-year overall survival was 50% in ESRD-RCC patients and 63% in RCC-only patients (p < 0.05).
Conclusion
More than 9 years with ESRD increased the risk of developing RCC 10-times compared to individuals without ESRD and the tumors showed a different histopathological pattern. Despite a less advanced tumor stage at diagnosis, the overall survival in ESRD-RCC patients was lower compared to patients with RCC-only.
Introduction
End-stage renal disease (ESRD) is a global health burden, with 564,638 affected individuals in Europe in 2016, equivalent to an overall prevalence of 823 patients per million population [Citation1]. ESRD is associated with high monetary expenses, rising risks of cardiovascular disease and cancer. Worldwide renal cell carcinoma (RCC) constitutes 2–3% of all malignancies and is the third most common urological cancer. The highest incidence of RCC is seen in western countries [Citation2,Citation3]. In 2018, ∼99,200 new cases of RCC were diagnosed in the EU and 39,000 patients died from the disease [Citation3,Citation4]. In Sweden, about 1,200 new cases of RCC are diagnosed each year. In 2016, the incidence per 100,000 persons was 15.8 in men and 8.2 in women [Citation4–6]. It has previously been shown that patients with ESRD have an increased risk of RCC, independent of the underlying cause of ESRD [Citation7–10]. Shrewsbury et al. [Citation11] estimated that around 2–7% of patients with ESRD develop RCC. Approximately 20% of patients with chronic kidney disease (CKD) who are treated with dialysis end up having acquired cystic kidney disease (ACKD) [Citation11,Citation12]. These patients have an increased risk of developing RCC and in clinical settings it has been described that 5% who undergo transplantation after dialysis develop RCC in their native kidneys [Citation8,Citation13–15]. Nevertheless, robust data is lacking since most studies have been performed on compiled selected patient materials without controls [Citation10,Citation11,Citation16,Citation17]. It may be useful to estimate the risk of developing RCC after various times with ESRD in a real-world setting in order to design screening programs for this patient group. The Swedish healthcare registers are of high quality and have high coverage [Citation18]. This case control study was designed based on these registers, including the whole Swedish population, with the aim to evaluate the risk of developing RCC in the ESRD population and to further characterize this cohort of ESRD patients diagnosed with RCC.
Materials and methods
Study population
This population-based study was designed to investigate patients with ESRD and subsequent RCC diagnosis (ESRD-RCC). ESRD was defined as chronic kidney disease 5 (CKD 5) or treatment with dialysis or kidney transplantation 0–40 (median 9) years before the diagnosis of RCC.
In total, 9,416 patients with RCC were identified in the National Swedish Kidney Cancer Register (NSKCR) from 2005 until 2014 [Citation19]. For every case 10 controls from the Swedish Population Register were identified, matched on gender, year of birth and county of residence. The controls were free of RCC at the end of the year of diagnosis of the index case. A control was allowed to become a case if RCC was diagnosed after the date of diagnosis of the index case. The cases and controls were linked to the Swedish Renal Registry (SRR), the National Patient Register (NPR) and the Cause of Death Register within the Renal Cell Cancer Database Sweden (RCCBaSe), as described earlier [Citation18]. All data in this study are extracted from RCCBaSe. The ESRD patients were searched for in SRR and in the NPR using the ICD10 codes N185 (chronic kidney disease, stage 5), Z49 (kidney dialysis) and Z94.0 (kidney transplanted). The first time the patient appeared in the SRR or either code appeared in NPR it was registered.
For calculations of time with ESRD and survival, the date of RCC diagnosis was used for the cases and for the controls the date of diagnosis of their corresponding RCC case.
The project was approved by the Ethics Review Board of Northern Sweden.
Statistical methods
Statistical analyses were carried out with Stata/IC 16.1 [Citation20]. Conditional logistic regression was used and odds-ratios (OR) with a 95% confidence interval were calculated. Overall survival difference between RCC patients and controls with and without ESRD was compared by Kaplan–Meier survival analysis and tested by the log rank method. Relative survival was calculated using the Ederer II method [Citation21]. Mortality data for the general population in Sweden was used to estimate expected survival rates for the studied populations. The mortality data comprised the probability of death for single-year age groups and gender in 1-year calendar periods. Differences of excess mortality rate were assessed via Poisson regression and use of the strs module for Stata [Citation22]. Excess mortality rate ratios with 95% confidence intervals were calculated. The association of survival with ESRD, age, sex, tumor diameter, tumor stage and occurrence of metastases and tumor morphology were assessed by uni- and multivariable Cox proportional hazard regression analysis. Hazard ratios (HR) with 95% confidence intervals were calculated. The proportional-hazards assumption was tested on the basis of Schoenfelds residuals after fitting a Cox regression model. It was violated (p < 0.05) for some of the covariates, and, thus, their HR must be interpreted as an average over the time studied. Data is presented as numbers and percentages or median and interquartile range. Median differences were calculated with Wilcoxon rank-sum test. Frequency distributions between categorical variables were compared with Fisher's exact or Chi square test as appropriate. All statistical tests were two-sided, and a p-value of < 0.05 was considered statistically significant.
Results
Patient characteristics
The study included 117 patients with ESRD-RCC. They were compared with 9,299 patients with RCC-only and 264 controls with ESRD and 93,895 without ESRD. Of the 117 ESRD-RCC patients, 69 (59%) were transplanted before the RCC diagnosis compared to 98 patients (37%) among 264 ESRD controls (p < 0.001). The median time from onset of ESRD to the date of RCC was 8.5 years. The corresponding time for the controls was 1.6 years ().
Table 1. Patient characteristics and time in ESRD.
Occurrence of RCC in the ESRD population
There was a statistically significant increased risk for RCC, with an odds ratio (OR) of 4.5 (95% CI = 3.6–5.6, p < 0.001), when ESRD patients were compared with those without ESRD. With less than 9 years from RCC diagnosis, the risk was 2.9-times higher (95% CI = 2.2–3.9, p < 0.001) and with more than 9 years from diagnosis, the risk was more than 10-times higher (OR = 10.2, 95% CI = 7.0–14.8, p < 0.001). The 9-year cut-off was chosen as it was the median time with diagnosed ESRD to the date of primary diagnosis of RCC.
Comparison of RCC with and without ESRD
When comparing ESRD-RCC patients with RCC-only, the median age at diagnosis was 65 vs 68 years, median tumor diameter was smaller (4.5 vs 6.0 cm), fewer were high stage, 13% vs 19% were M1. Tumor morphology revealed 53% vs 78% clear cell RCC, 30% vs 11% papillary RCC and 8% vs 5% chromophobe RCC in ESRD-RCC and RCC-only patients, respectively (). There were significant differences in the distribution of clear cell RCC vs papillary RCCs, clear cell RCC vs chromophobe RCC and papillary RCC vs chromophobe RCC, between ESRD-RCC and RCC-only patients (p < 0.001, p = 0.020, and p = 0.002, respectively).
Table 2. RCC patient and tumor characteristics.
Survival
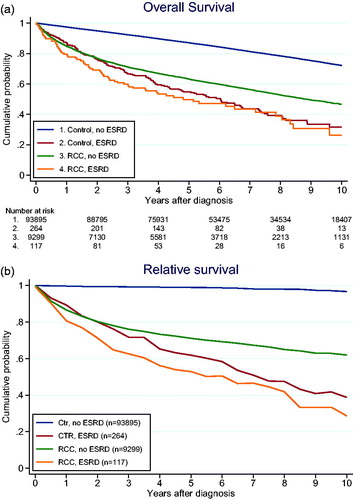
The 5-year overall survival (OS) was 50% (95% CI = 40–59%) in patients with ESRD-RCC and 63% (62–64%) in RCC-only patients (p < 0.001). The corresponding 10-year OS was 26% (15–40%) and 47% (45–48%), respectively (p < 0.001). The OS-curves were similar between ESRD patients with or without RCC (). At the same time RCC-ESRD patients were younger (median 65 vs 68 years, p < 0.001) and when differences in expected survival were taken into account by calculating relative survival and analyzing excess mortality rate ratio the difference was still non-significant (p = 0.27) ().
Risk factors for death in RCC-patients
Univariable analysis identified several risk factors for worse OS, including: patient age, T-stage, M-stage at diagnosis, different morphology and ESRD before RCC. In a multivariable Cox regression analysis age, T-stage, M-stage and collecting duct carcinoma remained negative factors for OS, while chromophobe histology was positive for OS. ESRD before RCC diagnosis remained an independent negative predictor of OS with a HR of 2.44 (95% CI = 1.84–3.26, p < 0.001) ().
Table 3. Uni- and multivariable Cox-regression, 5-year overall mortality, for RCC patients.
Discussion
Our nationwide population-based case-control study shows that ESRD have an increased risk of developing RCC in their native kidneys, confirming previous studies [Citation8–11,Citation16,Citation17]. ESRD increased the risk to develop RCC 3–10-times depending on the length of time with ESRD. After 9 years with ESRD the risk of RCC was 10-times higher compared with matched controls.
In the present study the patients with ESRD and subsequent RCC had significantly smaller tumors with lower stage at the time of detection compared with RCC-only patients. This is in line with Neuzillet et al. [Citation10], who reported the largest series of RCC in ESRD patients and showed a predominance of small, low-stage and low-grade tumors. Like others have suggested previously, one reason might be that ESRD patients undergo more medical investigations including CT/ultrasound-screening than the general population and therefore these tumors are identified earlier and are smaller at detection [Citation23,Citation24]. However, this earlier detection cannot explain the difference of morphological patterns between the RCC-only patients and the patients with ESRD-RCC. In the group of patients with ESRD-RCC we found significantly more papillary and chromophobe RCCs. Our results are consistent with the histopathological distributions that have been previously reported [Citation8,Citation9,Citation14,Citation15,Citation17,Citation25]. The main hypothesis for explaining the RCC type in ESRD patients is based on the fact that ACKD incidence increases over time, with dialysis reaching almost 90% over 5–10 years [Citation9,Citation13,Citation24]. Tsuzuki et al. [Citation9] produced a comprehensive review that discusses the epidemiology of RCC arising in ESRD patients. They noticed two specific RCC types after long-term dialysis (>10 years): Acquired cystic disease-associated RCC and clear cell papillary RCC, in contrast to our RCC morphological subtypes. These tumors had a more indolent clinical course, but they also observed that long-term dialysis worsens the prognosis for ESRD-RCC patients [Citation26]. There are arguments to support specific oncogenic and growth factor mechanisms in ESRD-RCCs, but the mechanisms are not fully understood and need further investigation. Hypertension is a risk factor for both ESRD and RCC and is common in ESRD-patients. In theory this might also contribute to the increased incidence of RCC in ESRD [Citation27].
Despite having less advanced tumors at diagnosis, we showed that the OS in ESRD-RCC patients is significantly lower compared with RCC-only patients. In previous studies, ESRD-RCC patients showed a more favorable cancer-specific survival, compared with RCC patients only [Citation8–11,Citation28]. From a patient perspective, cancer specific survival only is less relevant for the ESRD-RCC patients since it does not capture the decreased survival due to ESRD. We have therefore used relative survival, which is a measure of excess mortality, and OS in the comparisons between groups. In multivariable analysis, ESRD-RCC was a negative prognostic factor for OS with a HR of 2.44. We also observed that the control ESRD patients without RCC had a similar OS outcome as ESRD-RCC patients. When adjusting for age and other causes of death by analyzing relative survival, we still did not observe any significant difference in survival (p = 0.27). Song et al. [Citation17] constituted a comparative analysis of survival outcome between 362 RCC-only and 181 ESRD-RCC patients. They observed a decreased cancer-specific survival and OS for the ESRD-RCC patients. Likewise, Chen et al. [Citation29] noticed in a retrospective study that the OS for ESRD-RCC patients was decreased compared to RCC only. Our data is the first to compare ESRD-RCC patients with controls with and without ESRD. Patients with ESRD are known to have a shorter life expectancy than the general population, depending on the age at diagnosis. The expected life span after ESRD-onset is approximately one-third compared to the age-matched general population [Citation1,Citation30]. The survival outcome corresponds with our results, one can therefore argue that it is the ESRD that causes the decreased life expectancy not the RCC. Subsequently, the RCC caused by ESRD is described to be a more indolent and less aggressive disease [Citation8,Citation10,Citation11]. ESRD patients with RCC may belong to a subgroup with less comorbidity. Hence, they live longer with ESRD, which in time induces the RCC. The real effect of the RCC on survival is thus masked when the two groups are compared. The increased OR for RCC with time supports this explanation.
Limitations of the study are the retrospective register-based design, despite high coverage of the registers, lacking more detailed clinical data. The strength is that it is an unselected population-based cohort with a 99% coverage of the national population. Also, to our knowledge it is the first study investigating this issue with matched controls. A prerequisite for our study has been the high-quality health registers in Sweden and the unique research data base, RCCBaSe [Citation18].
Although the whole of Sweden is covered, the ESRD patients with and without RCC are currently too few to study other risk factors for RCC than time in ESRD.
Conclusion
This population-based case-control study shows that patients with ESRD have a 5–10-times increased time-dependent risk of developing RCC. Furthermore, ESRD patients have smaller tumors with lower stage at the time of detection compared to RCC patients without ESRD. Histopathologically there were more frequently papillary and chromophobe RCCs in the ESRD group. Nevertheless, the overall survival in ESRD-RCC patients is significantly lower compared to RCC patients without ESRD, probably due to the detrimental effect of the ESRD. In our multivariable analysis ESRD is shown to be an independent predictor of mortality with a hazard ratio of 2.44.
Ethical approval
The study was performed after approval of the regional Ethic review board of northern Sweden. Dnr: 2012-418-31M and 2014-301-32M.
Acknowledgements
Many thanks to the NSKCR steering panel for the continuous work with the National Swedish Kidney Cancer Register. Moreover, the authors are grateful to the people and the work behind RCCBaSe and their register-design and setting in the database. Lastly, a special thanks to Magnus Öberg at Registercentrum Norr for skillful data management of the database.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Kramer A, Pippias M, Noordzij M, et al. The European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2016: a summary. Clin Kidney J. 2019;12(5):702–720.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924.
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387.
- National Swedish Kidney Cancer Register, 2020 update. Available from: https://www.cancercentrum.se/samverkan/cancerdiagnoser/urinvagar/njurcancer/kvalitetsregister/.
- Thorstenson A, Bergman M, Scherman-Plogell AH, et al. Tumour characteristics and surgical treatment of renal cell carcinoma in Sweden 2005–2010: a population-based study from the national Swedish kidney cancer register. Scand J Urol. 2014;48(3):231–238. Jun
- Thorstenson A, Harmenberg U, Lindblad P, et al. Cancer characteristics and current treatments of patients with renal cell carcinoma in Sweden. Biomed Res Int. 2015;2015:456040.
- Hashimoto Y, Takagi T, Kondo T, et al. Comparison of prognosis between patients with renal cell carcinoma on hemodialysis and those with renal cell carcinoma in the general population. Int J Clin Oncol. 2015;20(5):1035–1041. Oct
- Breda A, Lucarelli G, Rodriguez-Faba O, et al. Clinical and pathological outcomes of renal cell carcinoma (RCC) in native kidneys of patients with end-stage renal disease: a long-term comparative retrospective study with RCC diagnosed in the general population. World J Urol. 2015;33(1):1–7.
- Tsuzuki T, Iwata H, Murase Y, et al. Renal tumors in end-stage renal disease: a comprehensive review. Int J Urol. 2018;25(9):780–786.
- Neuzillet Y, Tillou X, Mathieu R, et al. Renal cell carcinoma (RCC) in patients with end-stage renal disease exhibits many favourable clinical, pathologic, and outcome features compared with RCC in the general population. Eur Urol. 2011;60(2):366–373.
- Shrewsberry AB, Osunkoya AO, Jiang K, et al. Renal cell carcinoma in patients with end-stage renal disease has favorable overall prognosis. Clin Transplant. 2014;28(2):211–216.
- Van Poppel H, Nilsson S, Algaba F, et al. Precancerous lesions in the kidney. Scand J Urol Nephrol Suppl. 2000; 34(6):136–165.
- Ishikawa I, Saito Y, Asaka M, et al. Twenty-year follow-up of acquired renal cystic disease. Clin Nephrol. 2003;59(3):153–159.
- Kondo T, Sasa N, Yamada H, et al. Acquired cystic disease-associated renal cell carcinoma is the most common subtype in long-term dialyzed patients: Central pathology results according to the 2016 WHO classification in a multi-institutional study. Pathol Int. 2018;68(10):543–549.
- Schwarz A, Vatandaslar S, Merkel S, et al. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. CJASN. 2007;2(4):750–756.
- Gigante M, Neuzillet Y, Patard JJ, et al. Renal cell carcinoma (RCC) arising in native kidneys of dialyzed and transplant patients: are they different entities? BJU Int. 2012;110(11b):E570–E573.
- Song C, Hong SH, Chung JS, et al. Renal cell carcinoma in end-stage renal disease: multi-institutional comparative analysis of survival. Int J Urol. 2016;23(6):465–471.
- Landberg A, Lindblad P, Harmenberg U, et al. The renal cell cancer database Sweden (RCCBaSe) – a new register-based resource for renal cell carcinoma research. Scand J Urol. 2020;54(3):235–240.
- Landberg A, Bruce D, Lindblad P, et al. Validation of data quality in the National Swedish Kidney Cancer Register. Scand J Urol. 2021;1–7.
- StataCorp. Stata: release 16. Statistical software. College Station (TX): TSCL; 2019.
- Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121.
- Ce DP. Estimating and modeling relative survival. Stata J. 2015;15(1):186–215.
- Hurst FP, Jindal RM, Fletcher JJ, et al. Incidence, predictors and associated outcomes of renal cell carcinoma in long-term dialysis patients. Urology. 2011;77(6):1271–1276.
- Karami S, Yanik EL, Moore LE, et al. Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant. 2016;16(12):3479–3489. Dec
- Billis A, Freitas LLL, Costa LBE, et al. Genitourinary malignancies in transplant or dialysis patients: the frequency of two newly described 2016 World Health Organization histopathologic types. Transplant Proc. 2017;49(8):1783–1785. Oct
- Goh A, Vathsala A. Native renal cysts and dialysis duration are risk factors for renal cell carcinoma in renal transplant recipients. Am J Transplant. 2011;11(1):86–92.
- Hidayat K, Du X, Zou SY, et al. Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. 2017;35(7):1333–1344. Jul
- Klein JA, Gonzalez SA, Fischbach BV, et al. Routine ultrasonography surveillance of native kidneys for renal cell carcinoma in kidney transplant candidates. Clin Transplant. 2016;30(8):946–953.
- Chen K, Huang HH, Aydin H, et al. Renal cell carcinoma in patients with end-stage renal disease is associated with more favourable histological features and prognosis. Scand J Urol. 2015;49(3):200–204.
- van Walraven C, Manuel DG, Knoll G. Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis. 2014;63(3):491–499.