Abstract
Aim
To evaluate the use of repeat transurethral resection of the bladder (reTURB) in stage T1 bladder cancer and its impact on treatment and survival in a Norwegian population-based cohort.
Material and methods
1130 patients registered at the Cancer Registry of Norway between 2008 and 2012 with primary urothelial T1 cancer were included. Information on surgical and medical procedures was provided by the Norwegian Patient Registry. Descriptive statistics were used to evaluate characteristics of patients receiving reTURB or not within 12 weeks from primary TURB (primTURB). Survival models identified risk factors and estimated cause-specific survival rates (CSS) adjusted for sex, age, WHO grade, concomitant cis and detrusor muscle at primTURB and treatment.
Results
The 648 (57%) T1 patients with reTURB were significantly younger and had more WHO high grade tumors compared to those without reTURB. Of 275 patients without detrusor muscle at primTURB 114 (41%) had no reTURB. Of reTURB patients, 45 (7%) had muscle invasive tumor, 110 (17%) T1 and 378 (58%) Ta, cis or T0. Two-thirds of 81 patients receiving early cystectomy after reTURB had T1 or muscle invasive bladder cancer at reTURB. ReTURB did not impact adjusted CSS, but patients with T1 at reTURB had significantly lower CSS than those with < T1 conditions.
Conclusions
Almost half of the T1 patients did not undergo reTURB as recommended in guidelines. We show that reTURB makes the histology result more reliable with impact on both treatment and survival. Our results support the use of reTURB as recommended by EAU guidelines.
1. Introduction
T1 bladder cancer is an invasive tumor, infiltrating the lamina propria, but not the detrusor muscle of the bladder wall. T1 tumors account for about 15–20% of bladder cancer cases at diagnosis and are mainly WHO/2004 high grade tumors [Citation1]. The tumor is removed by a transurethral resection of the bladder (TURB) [Citation2]. In the diagnosis of T1 tumors, the base of the tumor should be submitted for histological examination separately from the exophytic tumor to secure demonstration of detrusor muscle in the specimen. Lack of muscle has been shown to increase the risk of overlooking an existing muscle invasive bladder tumor and is associated with an increased number of recurrences [Citation3–5]. Tumor tissue remaining after the primary TURB (primTURB) has been reported in 33–78% of the histology reports obtained by a repeat TURB (reTURB) with upstaging to muscle invasive bladder cancer in about 8% of cases [Citation6,Citation7]. In order to increase the diagnostic accuracy in patients with a T1 tumor and to ensure an early diagnosis of a muscle invasive tumor, the European Association of Urology (EAU) guidelines have since 2008 recommended a reTURB after primTURB in patients with a newly diagnosed T1 tumor and not eligible for immediate cystectomy [Citation8]. Further, the demonstration of detrusor muscle in the TURB specimen is viewed an obligate quality indicator in the management of T1 tumors [Citation1,Citation9,Citation10].
To our knowledge, this is the first Norwegian population-based study describing the use of reTURB and its consequences for the treatment of T1 patients. We focus on the differences between the reTURB vs the no reTURB group as well as reTURB histology results and their consequences for early treatment and survival.
2. Material and methods
2.1. Material
2.1.1. Data sources
Both the Cancer Registry of Norway and the National Patient Registry provided data for the current study. The Norwegian Cancer Registry has since 1953, compulsory by law, registered virtually all new cancer diagnoses in Norway and receives information from three independent sources (clinicians, pathology laboratories, and from the Cause of Death Registry) [Citation11]. The Norwegian Patient Registry includes administrative data on all treatment patients have received at all hospitals in Norway. Patients were identified through the personal identification number assigned to all citizens of Norway.
2.1.2. Study population
Based on morphological Snomed CT for transitional cell carcinoma of the urinary bladder, we identified 1389 patients with a first lifetime T1 urothelial carcinoma of the urinary bladder diagnosed by TURB between 2008 and 2012 (and no muscle invasive bladder tumor diagnosed prior to the T1 diagnosis). As a recent cancer diagnosed prior to the T1 diagnosis might potentially influence both the choice of treatment and survival, we excluded 144 patients with another cancer diagnosed within 5 years prior to the T1 diagnosis. We also excluded 115 patients who were not eligible for reTURB, i.e. 76 patients undergoing immediate cystectomy (within 6 months after primTURB and without reTURB) and 39 patients who died within 3 months after the T1 diagnosis (Supplementary Figure 1).
For all 1130 patients included, we obtained Norwegian Patient Registry dates for medical and surgical procedures related to the bladder cancer diagnosis such as dates of primTURB, reTURB, as well as type and date of early treatment provided within 6 months after reTURB. A reTURB was defined as a second TURB within 12 weeks after the primTURB (without BCG instillation in between). The Norwegian Cancer Registry received histology reports at reTURB for 323 (50%) out of 648 patients. Missing histology reports were most likely the result of no report being mailed to the Cancer Registry when the specimen did not reveal any malignant tumor tissue or when no specimen was taken at reTURB e.g. only coagulation. We therefore assigned T0 as stage for the remaining 325 patients without a reTURB histology report (TX), and thus considered them as patients with a T0 reTURB stage in the main analysis. Thus, the group of 378 patients with the reTURB stage T0, comprised these patients and 53 patients with a T0 histology report. Early cystectomy was defined as cystectomy performed within 6 months after reTURB. Early BCG (Bacille Calmette Guérin) treatment was defined as a BCG treatment which started within 8 weeks after primTURB or reTURB if performed. As the coverage for the ATC code for BCG treatment was low, we defined BCG treatment either by the ATC code (L03AX03) or as the application of at least three subsequent intravesical treatments with not more than 15 days in between two instillations. Intravesical treatment given the same day of TURB was not registered as BCG. Our variable ‘early treatment’ comprises information on whether the patient had received early cystectomy, early BCG, or none of these treatments.
All histology reports related to primTURB and reTURB, as available at the Cancer Registry, were reviewed by the first author (AB) recording stage, WHO grade (WHO 2004) [Citation12,Citation13], the presence of concomitant cis and of detrusor muscle in the specimen. In survival analyses, we defined the grade as the highest grade reported among TURB and reTURB histologies. Likewise, we defined concomitant cis as reported if it was described in at least one of these histology reports.
2.2. Statistics
The patients were followed from the T1 diagnosis until death, migration, or end of follow up on the 30th of June 2017, whichever came first. The median follow-up time was 6.9 years. The last update of cause of death was on December 31st of 2016.
Descriptive statistics were used to describe patient characteristics, the use of reTURB, early treatment and survival of the study population, overall and stratified for reTURB. Unadjusted cause-specific survival (CSS) and overall survival (OS) were calculated by the Kaplan-Meier approach and survival differences were assessed by applying the Log-rank test. Flexible parametric survival models [Citation14] were applied in order to quantify risk factors for bladder cancer related death and to estimate adjusted survival curves (CSS). In addition to reTURB (yes/no), factors included in these models, were age, sex, concomitant cis, grade, detrusor muscle in the specimen and early treatment. We also estimated adjusted CSS stratified for reTURB histology results (no reTURB, T0/Ta/cis, T1, T2-4) within the same framework, thereby adjusting for age, sex, concomitant cis and grade. The baseline hazard in these flexible parametric models was modeled using 4 degrees of freedom (df) for the spline variables using the Stata command stpm2 [Citation15]. The quantities reported are the hazard ratios (HRs) including 95% confidence intervals (CIs) and corresponding p-values.
3. Results
3.1. Use of reTURB
Our study population comprised 1130 T1 patients evaluable for reTURB and treatment of their T1 tumor of whom 648 (57%) underwent a reTURB within 12 weeks. The use of reTURB remained stable across the years (2008: 56%; 2009: 54%; 2010: 60%; 2011: 62%; 2012: 56%). Out of 275 patients with no detrusor muscle in the primTURB specimen, 114 (41%) did not receive reTURB (). Compared to patients without, those with reTURB were younger (median 72 vs 78 years) and more likely to be diagnosed with high grade tumors (96% vs 83%). The distribution of gender and the presence of concomitant cis or muscle in the specimen was similar irrespective of the performance of reTURB.
Table 1. Characteristics for Norwegian T1 bladder cancer patients diagnosed in the period of 2008-2012, stratified for reTURB.
3.2. Histology at reTURB
Muscle invasive bladder cancer was identified at reTURB in 45 (7%) out of 648 patients with reTURB, T1 tumor in 110 (17%), and non-invasive or no malignancy (Ta, cis, T0) in 493 cases (76%) (). Out of 487 patients with reTURB and detrusor muscle in the primTURB specimen, 24 (5%) reTURB histologies showed muscle invasive tumors. In contrast, 21 cases of muscle invasive tumors (13%) were found in the 161 patients without muscle in the primTURB specimen. Further, 59% of the 487 patients with muscle in the primTURB specimen and 58% of the 161 patients without, had T0 at reTURB.
Table 2. Histology for T1 patients at reTURB dependent on tumor characteristics grade, muscle in the specimen and concomitant cis at primary TURB.
3.3. Early treatment after reTURB
Among the 648 patients with reTURB, 81 (13%) underwent early cystectomy () within 6 months. More were treated with early BCG (20% vs 7%) and fewer had no early treatment (67% vs 93%) compared to patients without reTURB. Half of the patients (22 out of 45) with a muscle invasive tumor at reTURB underwent early cystectomy and they were 8 years younger compared to those without cystectomy. Twenty-eight percent of the patients with T1 and 6% of patients with tumor stage < T1 at reTURB had early cystectomy.
Table 3. Early treatment, cystectomy within 6 months and BCG within 8 weeks, for patients with or without reTURB.
3.4. Survival and the impact of reTURB
Unadjusted 5-year OS was 69% for the reTURB group and 53% for patients without reTURB (p = 8.3·10−5) (). The corresponding CSS was 83% (reTURB) and 79% (no reTURB); (p = 0.019) ().
Figure 1. Observed cause-specific (A) and overall (B) survival including number of patients at risk for all T1 patients and stratified by the performance of reTURB.
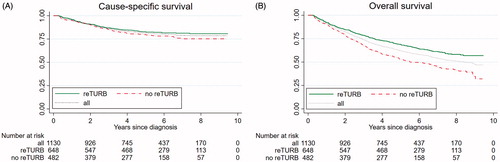
Overall, no difference in CSS was seen between patients with reTURB compared to those without (HR = 1.02; CI: 0.75–1.37; p = 0.933) when adjusting for age, sex, grade, concomitant cis and early treatment. In contrast, the adjusted OS was higher in patients with reTURB (vs no reTURB) (HR = 0.79; CI: 0.66–0.96; p = 0.017).
When investigating the risk factors for CSS, older age and the tumor stage at reTURB emerged as the major risk factors (). Neither grade nor concomitant cis influenced CSS significantly. When compared to patients with tumor stage < T1 (T0, Ta, cis) at reTURB in a post-hoc analysis, those with T1 and muscle invasive tumor at reTURB had a significantly higher risk of bladder cancer related death (T1: HR = 2.7; CI: 1.7–4.3; p = 2.6·10−5 and MIBC: HR = 7.2; CI: 4.2–12.4; p = 4.5·10−13). displays the corresponding adjusted CSS curves stratified by reTURB tumor stage. The 5-year CSS was estimated to be 87% (CI: 83–90%) for patients with T0, Ta or cis at reTURB, 69% (CI: 61–79%) for those with T1and 43% (CI: 32–57%) for patients with muscle invasive bladder cancer.
Figure 2. Adjusted cause-specific survival for patients with no reTURB and stratified for tumor stage in those with reTURB. MIBC: muscle invasive bladder cancer.
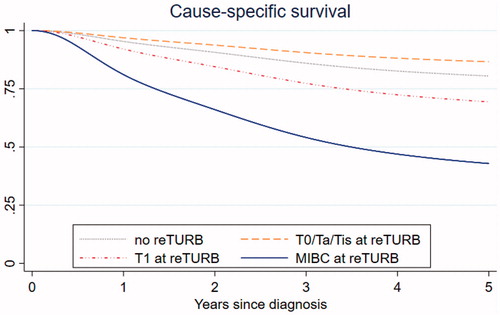
Table 4. Results from the cause-specific survival analysis adjusted for early treatment.
4. Discussion
This retrospective Norwegian population-based study comprised 1130 patients diagnosed with T1 bladder cancer in 2008–2012 investigated the use of reTURB and its consequences with respect to subsequent treatment and survival.
4.1. Frequency of reTURB
TURB represents a diagnostic procedure as well as the only surgical treatment for the majority of T1 patients. A T1 diagnosis requires the verification of tumor-free detrusor muscle in the histology report. The role of reTURB is to diagnose muscle invasive tumor not detected at primTURB and to identify and completely remove remaining or re-growing tumor tissue. Based on the medical literature before 2008 and the EAU guidelines since 2008 our percentage of reTURB (57%) is surprisingly low, though it is similar to the Swedish study on T1 patients diagnosed in 2008 and 2009 showing a reTURB rate of 55% [Citation16].
In our study, younger patients, and patients with a high grade tumor at primTURB were more likely to have a reTURB whereas sex and concomitant cis were of no impact. Surprisingly, the lack of detrusor muscle in the primTURB specimen was not associated with the rate of reTURB even though multiple studies [Citation2,Citation4,Citation17] and the EAU guidelines since 2008 [Citation8] emphasized the importance of this finding for the risk of relapses and progression. Several other factors besides age and grade, such as tumor size, tumor multiplicity, and completeness of resection, which were not available to us, may have influenced the decision to perform or to omit a reTURB. High age alone hardly explains our low percentage of reTURB. A TURB is generally well tolerated even by elderly patients with a complication rate not exceeding 5% [Citation2,Citation18]. Other reasons for not performing reTURB could be unawareness of guideline recommendations or surgeons trusting the primary resection to be complete. Some patients without reTURB might also get an initial TURB for palliative purposes.
4.2. Post reTURB histology
Upstaging to muscle invasive bladder cancer was found in 7% of 648 patients at reTURB, which is in line with 7-8% reported in other studies [Citation7,Citation19]. The percentage of T1 tumors at reTURB was 17%, which is less than 31% reported by Gontero et al. [Citation17] and 43% reported by Patchan et al. in a Swedish cohort study[Citation16]. However, the percentage T1 at reTURB in Gontero et al’s study is not directly comparable to our result as the authors had excluded patients with muscle invasive tumor at reTURB. The tumor stage at reTURB contains important clinical information, e.g. a T1 tumor at reTURB indicates a 25–80% risk of progression into MIBC [Citation20–22]. In our study the risk of understaging an existing muscle invasive tumor increased by a factor of 2.6 (13% vs 5%) if the primTURB specimen lacked detrusor muscle. Importantly, our data showed that the demonstration of muscle in primTURB specimen did not entirely rule out a muscle invasive tumor at reTURB as 24 out of 487 (5%) patients with detrusor muscle in primTURB had muscle invasive bladder tumor at reTURB.
Fifty-eight percent of the patients were tumor-free at reTURB, which is higher than reported by Patchan et al. (35%) and Gontero (29%). A systematic review reported a range of 29–80% tumor-free reTURB histologies [Citation7]. In line with this variations of reported numbers, the Swedish report [Citation16] showed large inter-hospital variations of the number of reTURB. It must be emphasized that the histological results from primTURB and reTURB must be viewed on the background of the urologist’s experience and technical skills [Citation4,Citation23,Citation24]. Thus, it should be discussed whether at least more challenging TURB procedures e.g. in patients with multiple and/or large tumors and most reTURB procedures should be performed by experienced urologists in high-volume hospitals to secure the best possible diagnostic and therapeutic results.
4.3. Post reTURB treatment
About half of the patients with a muscle invasive tumor and about one third of those with a T1 tumor at reTURB underwent early cystectomy. We have previously shown [Citation25] that T1 patients <75 years as well as patients with high grade tumors or concomitant cis were most likely to undergo cystectomy within 6 months after primTURB or reTURB respectively. Additional factors for not performing or delaying cystectomy could have been severe comorbidity or the patient’s refusal. Both clinicians and patients should be aware that survival is unfavorable in some patients undergoing deferred cystectomy [Citation25,Citation26]. Moschini et al. even found increased mortality rates for T1 patients with cystectomy after progression to muscle invasive disease compared to mortality for primary muscle invasive bladder cancer [Citation27].
4.4. Survival
There is an ongoing discussion whether there is any survival benefit for patients undergoing reTURB [Citation5,Citation17]. When adjusting for relevant clinical factors, no significant difference of CSS was found for patients with vs without reTURB in our study. Gontero et al. showed that reTURB impacted CSS (and OS) but only for patients without DM in the primTURB [Citation17]. In line with our results, a Swedish report did not find an impact of the use of reTURB on survival when adjusting for clinical factors [Citation16]. However, we did find survival differences dependent on the depth of tumor infiltration demonstrated in the reTURB specimen. CSS was more favorable in T1 patients with a non-invasive tumor or tumor stage T0 at reTURB when compared to patients who had T1 at reTURB. These unfavorable result of stage T1 at reTURB might indicate a particularly high biological aggressivity, not reflected by routine histological results. Some T1 tumors might also be more challenging to remove completely e.g. because of their location, size and multifocality, thereby already initially identifying patients with an increased risk of progression. Finally, we consider the early identification of muscle invasive tumor and of ‘aggressive’ T1 tumors by a reTURB to be clinically important since the reTURB histology increases the scientific basis for consideration of early cystectomy. If cystectomy is not conducted, patients with T1 tumors at both primTURB and reTURB should at least be considered for frequent controls and/or an additional TURB.
4.5. Limitations and strengths
This retrospective registry-based study has several limitations. We assigned tumor stage T0 to patients with non-available histology report after reTURB (TX). All Norwegian pathology laboratories are required to transfer all histology reports showing a cancer diagnosis to the Norwegian Cancer Registry. Such transferals are not required in case of ‘no malignant tumor’ unless the patient had an earlier cancer diagnosis. We are confident that our T0 assignment is reasonable since the reporting of ‘no malignancy’ to the Cancer Registry easily could have been forgotten, especially if information of earlier cancer has not been provided by the surgeon. Thus, the 325 TX patients without histology report at reTURB most probably represented a positively selected sub-sample, with a higher frequency of T0 after reTURB than the 323 cases with a report available. This is also supported by the more favorable survival of patients without a reTURB histology report (TX) versus patients with T0/Ta/cis reTURB histology (Supplementary Figure 2). Moreover, the results are similar and the conclusions the same when treating TX as a separate group in the analysis (Supplementary Table 1). Another issue is the dependency of the results from the thresholds chosen for the definition of reTURB and early treatment after diagnosis. However, thresholds are always a simplification of a complex diagnostic workaday. With respect to the thresholds, we chose a liberal approach, allowing for delays in the diagnostic and clinical workup. Thus, although the EAU-guideline recommends reTURB within 4–6 weeks, we defined a TURB within 12 weeks as a reTURB. We also defined early treatment as BCG treatment within 8 weeks and cystectomy within 6 months ensuring that we capture all patients with the intention of reTURB and early treatment that for some reason were delayed. For a few patients, these treatments could therefore represent a consequence of a relapse.
We do not have information about size and multiplicity of the T1 tumors or of comorbidities of the T1 patients. Further, comparison between reTURB and no reTURB patients must be interpreted with caution since these groups might be different with respect to several factors not available for assessment and these factors probably bias our results.
4.6. Conclusion
In Norway, almost half of the T1 patients diagnosed between 2008 and 2012 and almost half of the patients without detrusor muscle in the primTURB specimen did not undergo reTURB as recommended in the EAU guidelines. Our results support that both the early treatment after a T1 diagnosis and T1-related survival are dependent on the correct histological evaluation of the tumor, which is improved by the performance of a reTURB and support the use of reTURB as recommended by the EAU guidelines.
Author contributions
BKA conceived, coordinated, and designed the study, performed the statistical analysis, and drafted the manuscript together with AB and SF. RB contributed to the data management. AB was responsible for the clinical assessment of the patients included and, together with SF, contributed in the interpretation of the results, background knowledge on bladder cancer and writing the manuscript. All authors participated in writing of the manuscript and revised it critically. All authors have read and approved the final version of the manuscript.
| Abbreviations | ||
| CI | = | confidence interval |
| BCG | = | Bacille Calmette Guérin |
| cis | = | carcinoma in situ |
Supplemental Material
Download Zip (220.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol. 2019;76(5):639–657.
- Herr HW, Donat SM. Quality control in transurethral resection of bladder tumours. BJU Int. 2008;102(9 Pt B):1242–1246.
- Dutta SC, Smith JA Jr, Shappell SB, et al. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J Urol. 2001;166(2):490–493.
- Mariappan P, Zachou A, Grigor KM. Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol. 2010;57(5):843–849.
- Soria F, Marra G, D’Andrea D, et al. The rational and benefits of the second look transurethral resection of the bladder for T1 high grade bladder cancer. Transl Androl Urol. 2019;8(1):46–53.
- Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol. 1999;162(1):74–76.
- Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2018;73(6):925–933.
- Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54(2):303–314.
- Mostafid H, Brausi M. Measuring and improving the quality of transurethral resection for bladder tumour (TURBT). BJU Int. 2012;109(11):1579–1582.
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021–1029.
- Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer (Oxford, England: 1990). 2009;45(7):1218–1231.
- Lopez-Beltran A, Montironi R. Non-invasive urothelial neoplasms: according to the most recent WHO classification. Eur Urol. 2004;46(2):170–176.
- Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22(12):1435–1448.
- Crowther MJ, Lambert PC. A general framework for parametric survival analysis. Statist Med. 2014;33(30):5280–5297.
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265–290.
- Patschan O, Holmang S, Hosseini A, et al. Second-look resection for primary stage T1 bladder cancer: a population-based study. Scand J Urol. 2017;51(4):301–307.
- Gontero P, Sylvester R, Pisano F, et al. The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette-Guerin. BJU Int. 2016;118(1):44–52.
- Nieder AM, Meinbach DS, Kim SS, et al. Transurethral bladder tumor resection: intraoperative and postoperative complications in a residency setting. J Urol. 2005;174(6):2307–2309.
- Divrik RT, Sahin AF, Yildirim U, et al. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol. 2010;58(2):185–190.
- Palou J, Pisano F, Sylvester R, et al. Recurrence, progression and cancer-specific mortality according to stage at re-TUR in T1G3 bladder cancer patients treated with BCG: not as bad as previously thought. World J Urol. 2018;36(10):1621–1627.
- Herr HW, Donat SM. A re-staging transurethral resection predicts early progression of superficial bladder cancer. BJU Iin. 2006;97(6):1194–1198.
- Bishr M, Lattouf JB, Latour M, et al. Tumour stage on re-staging transurethral resection predicts recurrence and progression-free survival of patients with high-risk non-muscle invasive bladder cancer. CUAJ. 2014;8(5–6):306–310.
- Brausi M, Collette L, Kurth K, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41(5):523–531.
- Jancke G, Rosell J, Jahnson S. Impact of surgical experience on recurrence and progression after transurethral resection of bladder tumour in non-muscle-invasive bladder cancer. Scand J Urol. 2014;48(3):276–283.
- Blindheim A, Fosså S, Babigumira R, et al. T1 bladder cancer in Norway: treatment and survival. Scand J Urol. 2020;12:1–6.
- Denzinger S, Fritsche HM, Otto W, et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur. Urol. 2008;53(1):146–152.
- Moschini M, Sharma V, Dell’oglio P, et al. Comparing long-term outcomes of primary and progressive carcinoma invading bladder muscle after radical cystectomy. BJU Int. 2016;117(4):604–610.
