Abstract
Background
Transrectal prostate biopsy (TRbx) transfers colonic bacteria into prostatic tissue, potentially causing infectious complications, including sepsis. Our objective was to determine whether biopsy needle shape, surface properties and sampling mechanism affect the number of bacteria transferred through the colon wall, and evaluate a novel needle with improved properties.
Methods
The standard Tru-Cut biopsy needle used today was evaluated for mechanisms of bacterial transfer in a pilot study. A novel Tru-Cut needle (Forsvall needle prototype) was developed. TRbx was simulated using human colons ex-vivo. Four subtypes of the prototype needle were compared with a standard Tru-Cut needle (BARD 18 G). Prototype and standard needles were used to puncture 4 different colon specimens in 10 randomized sites per colon. Needles were submerged into culture media to capture translocated bacteria. The media was cultured on blood agar and then the total amount of transferred bacteria was calculated for each needle. The primary outcome measure was the percent reduction of bacteria translocated by the prototype needles relative to the standard needle. Secondary outcome measures were the effects of tip design and coating on the percent reduction of translocated bacteria.
Results
Prototype needles reduced the number of translocated bacteria by, on average, 96.0% (95% confidence interval 93.0-97.7%; p < 0.001) relative to the standard needle. This percent reduction was not significantly affected by prototype needle tip style or surface coating.
Conclusions
The Forsvall needle significantly reduces colonic bacterial translocation, suggesting that it could reduce infectious complications in prostate biopsy. A clinical trial has been initiated.
Background
Prostate cancer is mainly diagnosed and monitored using tissue obtained by a Tru-Cut biopsy needle [Citation1]. Transrectal prostate biopsy (TRbx) is the most common biopsy method [Citation1]. In TRbx, the biopsy needle transfers colonic bacteria through the rectal wall into the prostate and periprostatic tissue, potentially causing infectious complications, including sepsis [Citation1–3]. Currently, the risk of post-biopsy infection is 2–10% [Citation1,Citation4]. Although the use of pre-biopsy MRI, nomograms and biomarkers has reduced the need for prostate biopsies, huge numbers of men still undergo a prostate biopsy. Antibiotic prophylaxis reduces the risk of post-biopsy infection and is routinely used, but antimicrobial resistance has led to increasing rates of infections [Citation1,Citation5]. Current recommendations of antibiotic prophylaxis vary [Citation1,Citation6]. Escherichia coli (E. coli) is by far the most common bacterium isolated from patients with post-TRbx sepsis [Citation7].
In addition to standard antibiotic prophylaxis, methods to reduce infections after prostate biopsy include rectal swab cultures to individualize antibiotic prophylaxis, pre-biopsy rectal cleansing, and using the transperineal route for biopsy. However these methods are expensive, time consuming, introduce new side effects, or highly uncomfortable [Citation1,Citation8]. We are unaware of any attempts to reduce infectious complications by modifying the needle design or surface properties, even though infections are caused by the needle pushing bacteria into the tissue [Citation3]. Our hypothesis is that if bacterial transfer can be minimized, the risk of infection will decrease. In this study, we first aimed to examine physical properties of the standard Tru-Cut needle that can lead to bacterial transfer. We thereafter developed a needle with physical and mechanical properties designed to reduce the number of bacteria (bacterial load) transferred across the rectal wall to the prostate. Finally, we evaluated the needle’s performance in an ex-vivo study.
Materials and methods
Pilot study
The mechanism of action of the standard Tru-Cut needle is described in . We hypothesized that the Tru-Cut needle's forward-facing opening and rough, highly echogenic part may facilitate collection and deposition of bacteria into the tissue. To test this hypothesis, we conducted a pilot study using steel rods with grinded tips to simulate a closed needle with perfect fit between the needle parts. These rods are referred to as rod prototype needles. We punctured a simulated colon wall consisting of bovine meat covered with faeces from a healthy human and the number of translocated bacteria were measured. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) was used to determine the most common types of bacteria species translocated by the needles. Method details are in supplement A.
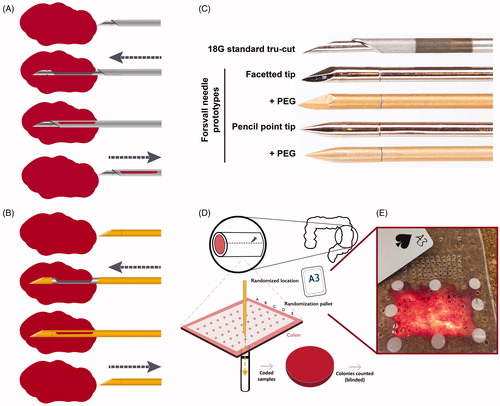
Figure 1. Ex vivo biopsy simulation method with standard needle and Forsvall needle prototypes. (A) Schematic depiction of standard Tru-Cut biopsy needle mechanism. (B) Schematic depiction of Forsvall needle biopsy mechanism. (C) The five needles used in the ex vivo simulation were a standard 18 G Tru-Cut needle (Bard) and four versions of the Forsvall 18 G stainless steel needle prototype: Facetted tip with no coating, facetted tip with gold + PEG coating, pencil point tip with no coating, and pencil point tip with gold + PEG coating. Note that the opening of the Tru-Cut needle is in the direction of travel, the opening of the Forsvall needle is in a 90-degree angle to the direction of travel. In the ex-vivo simulation the needles were used in this same order until all punctures were completed. (D) Schematic depiction of the experimental workflow. First, fresh colons were obtained after colon cancer surgery and opened to form a 5 × 8cm rectangle. This rectangular piece of colon was then mounted on a special bracket with the mucosal side up. A randomization pallet consisting of 50 evenly spaced holes labelled A–E and 1–10 was mounted over the colon. A card was drawn from a randomization deck of playing cards with labels corresponding to the holes on the randomization pallet. The corresponding hole was then punctured with the next needle in the series. The needle was immediately submerged in a tube filled with culture media to a depth of 6 cm, opened and closed to dislodge any collected material, and then removed. The culture media was plated on a code-labelled blood agar plate and incubated overnight, and the number of colonies was counted blindly to avoid bias. (E) Photograph of a colon mounted on the bracket with the randomization pallet placed on top, as viewed from above.
Forsvall needle prototype
Based on the results of the pilot study, we constructed an operational two-part biopsy needle with a closed tip, and a semi-automatic biopsy gun mechanism designed to force the two parts of the needle together, resulting in a smooth needle surface (). The resulting needle was named the Forsvall needle prototype after its inventor, hereafter referred to as the prototype needle.
We tested four versions of the prototype needle: facetted or pencil point tip, both with and without PEG (Polyethylene Glycol) coating. PEG may inhibit bacterial adherence to surfaces and is already safely used in humans in drug delivery systems [Citation9] and as a rectal spacer during prostate cancer radiotherapy [Citation10]. The PEG prototype needles were coated with a ∼100 nm gold film, to which PEG (10 kg/mol) was covalently bonded by 50 min soaking in 0.3 g/L PEG solution [Citation11].
Ex vivo randomized study
We used an ex-vivo human colon model to simulate bacterial transfer in TRbx. We compared the four prototype needles () to each other and to a common Tru-Cut needle (BARD 18 Gauge, 20 cm Tru-Cut needle for MAGNUM biopsy gun; referred to as the standard needle).
Patient material
The study was approved by the Ethical Review Board in Lund (Dnr: 2016/273) and informed consent was obtained from all patients prior to surgery. We obtained four human colon specimens, immediately after they had been resected during surgery for colon cancer. Patient and colon characteristics are described in . Colons with any other disease, such as diverticulosis, were excluded. Removed colons were rinsed with water according to standard surgical procedures and the 5 cm of the colon furthest from the tumour was cut off and opened to form a rectangle of about 5 × 8 cm. Pericolic fat was removed. Care was taken to avoid contaminating the outside of the colon. The colon was mounted on a special bracket with the mucosal side up and slightly stretched to achieve equal tension across the section. No visible faecal matter was present. A randomization pallet with 50 evenly spaced holes labelled A-E and 1-10 () was mounted over the colon. Because colon tissue necrotizes after 4–6 h of ischaemia [Citation12], all experiments were finished within 3 h after colon removal.
Table 1. Baseline characteristics of patients and colon specimens.
Randomization
The five needles were used in the same order throughout the experiment, ensuring that each needle was used for every fifth puncture. The puncture site was randomized using 50 playing cards, each with a code corresponding to a hole in the randomization pallet. The deck was shuffled for 5 min and prior to each puncture the top card was drawn. The puncture was randomized to the corresponding location ().
Colon puncture and bacterial capture
We have observed that a 20 cm needle reaches a maximum depth of 6 cm into the body when obtaining TRbx. Therefore after each puncture, but before it was removed from the colon specimen, the needle was submerged to a depth of 6 cm into a tube containing 9.4 mL Brain Heart Infusion medium (BHI) that was held below the colon. While submerged, the needle was agitated, opened, and closed for 4 s to release any attached material. To ensure sterility between punctures, a new sterile Bard needle was used each time. The four prototype needles were sterilized by wiping, soaked twice in ethanol, rinsed twice with distilled water, and finally dried with a sterile cloth.
In systematic TRbx, 10–12 punctures are done with the same needle. To simulate a full TRbx procedure, we additionally did 10 subsequent punctures in one colon without sterilizing the needles in-between. A previously un-punctured section of colon 4 was used, and puncture sites were randomized as above. In this experiment we compared the standard needle to the PEG-coated pencil tip prototype needle.
Quantitative bacterial culture
The tubes with collected bacteria were vortexed to get a single-cell suspension of the bacteria, and 100 μL of each solution was plated on blood agar plates. The plates were coded and incubated overnight at 37 °C in 5% CO2. The number of colony-forming units (CFU) on each plate was counted blindly by a technician to prevent bias. The total number of bacteria translocated by each puncture was calculated from the CFU in 100 µL multiplied by the total volume of culture medium (9.4 mL) used to capture the bacteria. Each CFU was considered to originate from one viable bacterium [Citation13].
Statistical analysis
To compare the number of bacterial colonies translocated by each needle, we used generalized linear mixed-effects models with a negative binomial distribution and a random intercept at the individual colon level as our main statistical method. We did this because the number of bacterial colonies (count data) displayed over-dispersion and colons were punctured repeatedly, representing data non-independency. Model assumptions were checked using qq-normal plots and histograms on deviance residuals. Results are reported as a percent reduction and presented with 95% confidence intervals (95% CI) and p values below 0.05 were regarded as statistically significant. To compare the effect of subsequent punctures without sterilization of the needle in between, we used an unpaired t-test to compare the number of bacteria translocated per puncture by each needle. Statistical analyses were performed using Stata (Stata MP 16.1, StataCorp, Texas, USA).
Results
Pilot study
To determine whether the needles’ surface properties affect bacterial translocation during TRbx, we punctured a simulated colon with three standard Tru-Cut needles and 10 versions of the rod prototype needle (steel rods with grinded tips with different surface coatings). Light microscopy images of the standard Tru-Cut needles after puncture indicated that faecal matter was collected in the opening between the inner and outer needle and also on the rough hyperechogenic zone ( and ). The rod prototype needles with low bacterial count had no visible faecal matter collection ().
Figure 2. Effect of physical properties and needle coating on bacterial translocation in a pilot experiment. (A) Representative images of a standard Tru-Cut biopsy needle (Mermaid 18 G) after a puncture through a simulated colon. Left images show the gap between the inner and outer needle in a closed (top) and opened (bottom) state. Right image shows the rough, highly echogenic area, used for enhancing ultrasound reflection and visualization of the needle within the body in other types of biopsy. The highly echogenic area has no use in TRbx because a needle guide is used to locate the needle tip. Arrows indicate areas of visible faecal matter and tissue collection. (B) Gold + PEG coated rod prototype needle after puncture of a piece of meat covered in faecal matter, simulating the rectal wall. No foreign matter was visible at this magnification. (C) Number of bacterial colonies translocated when a piece of meat covered in faecal matter was punctured with three different standard Tru-Cut needles and ten versions of the rod prototype needle with different combinations of surface coatings. The average bacteria translocated by all three standard needles (pooled) was set to 100% and the percentage translocated by each rod prototype needle was calculated relative to this number.
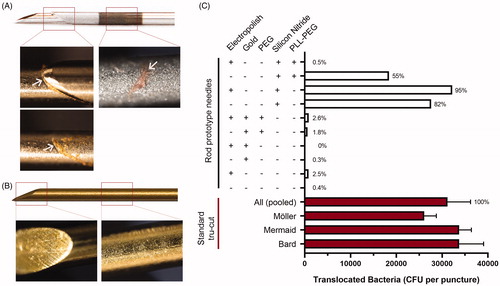
Uncoated, electropolished, gold-coated, and PEG-coated rod prototype needles translocated 98.7% less bacteria than the Tru-Cut needles (). Prototype needles coated with silicon nitride or PLL-g-PEG on silicon nitride did not reduce the number of translocated bacteria, indicating that this coating may have facilitated bacterial adhesion to the surface. MALDI-TOF MS analysis of 300 random colonies identified all 300 (100%) isolates to be E. coli.
Ex vivo randomized study
To determine whether the prototype needle can reduce bacterial translocation relative to the standard Tru-Cut needle, we tested fully openable versions of the prototype needle using ex vivo human colons to simulate TRbx as realistically as possible. We examined the needles by light microscopy and found that the standard Tru-Cut needle collected visible faecal matter in the forward-facing opening between inner and outer needles (), while the prototype needles did not collect any visible faecal matter ().
Figure 3. Standard Tru-Cut needle and prototype needle after puncture through an ex vivo human colon. (A) Representative images of the gap between the inner and outer needle parts of a standard 18 G Tru-Cut needle (Bard 18 G) when closed and partially open, following puncture of an ex-vivo human colon. White arrows indicate areas of visible fecal matter and tissue collection. Red line in the ‘open’ image indicates the position of the outer needle when closed, please note the extensive visual contamination. (B) Representative images of a 18 G Forsvall needle prototype (pencil tip with gold + PEG) following puncture with of an ex-vivo human colon, images show corresponding locations to images in A, closed and partially open. No foreign matter was visible at this magnification. (C) Mean number of colonies (CFU) translocated per puncture through each colon by the four Forsvall needle prototypes and the standard Tru-Cut needle. Percentages are indicated above each bar with the mean bacteria translocated by the standard needle set as 100% for each colon.
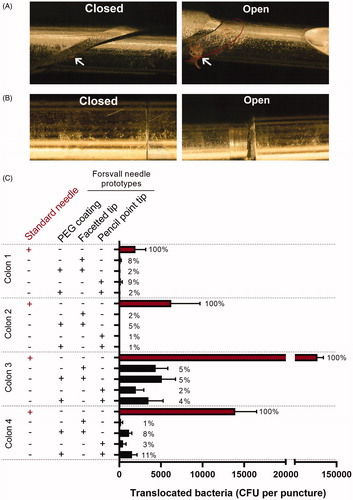
The prototype needles carried, on average, 96.0% fewer bacteria (95% CI: 93.0 to 97.7%, p < 0.0001) across the colon than the standard needle did (). The reduction in translocated bacteria was similar for all prototype needles regardless of tip style and the presence or absence of PEG coating (). Although the number of bacteria collected by the Tru-Cut needle differed between colons (, ), this did not affect bacterial reduction by the prototype needles (94-98% reduction for each colon, ).
Table 2. Percent reduction of translocated bacteria for each variation of the Forsvall prototype needle, as well as the four prototype needles grouped, relative to the standard Tru-Cut needle (BARD 18 Gauge 20 cm Tru-Cut biopsy needle for MAGNUM biopsy gun).
We also compared bacterial translocation by one prototype needle (pencil point tip and PEG coating) and the standard needle in 10 subsequent punctures without sterilization. The prototype needle carried 94.0% (p < 0.0001) fewer bacteria than the standard Tru-Cut needle across all punctures, which was similar to the 96% reduction we found when using the standard protocol.
Discussion
In this study, we explored the effect of biopsy needle design on bacterial translocation across the rectal wall in an experimental model, simulating TRbx. We identified design issues with the standard Tru-Cut needle related to bacterial translocation, and developed a novel needle with the aim to reduce bacterial translocation into the prostate at TRbx. Our results indicate that when a TRbx is performed, most of the bacteria that are transferred through the rectal wall are collected in the forward-facing gap between the inner and outer parts of the Tru-Cut needle (). The Tru-Cut needle design thus enables bacterial translocation and subsequent infection.
Figure 4. Schematic depiction of TRbx and bacteria/fecal matter deposition when using a standard tru cut needle (left) and the Forsvall needle prototype (right).
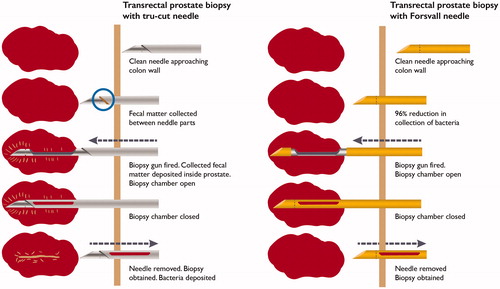
The Forsvall needle prototype significantly reduced bacterial translocation across the colon wall by 96% relative to the standard Tru-Cut biopsy needle. The two parts of the Forsvall needle are forced closed, resulting in a streamlined design without edges that minimizes the collection of foreign matter (bacteria and devitalized tissue) as the needle moves through the tissue. The results of our study indicate that the Forsvall needle may reduce the risk of post-TRbx infections. The association between bacterial load reduction and infection reduction has been shown in studies of pre-biopsy povidone iodine cleansing. In a quantitative bacterial study, extensive povidone-iodine cleansing reduced the bacterial load in the rectum by 97.2% and reduced the rate of infections from 4.3 to 0.6% [Citation8]. A meta-analysis showed that povidone-iodine cleansing reduced the risk of infections by 42% (95% CI 24–57%) [Citation14]. Although cheap, povidone-iodine cleansing may lead to allergic reactions, is time-consuming and uncomfortable for the patient, and its efficacy may be user dependent. Hence, this approach is not in widespread use. Our prototype needle reduced the translocated bacterial load to a similar extent. Compared with povidone-iodine, use of the Forsvall needle is not time-consuming, not expected to increase discomfort or side effects and is not user dependent. It also visually reduces translocation of devitalized tissue that may be a possible substrate for bacterial growth and later clinical infection. Needles with either facet or pencil point tip design, with or without PEG coating, were all equally efficient at reducing bacterial transfer indicating that the smooth needle surface, achieved through the closed needle design and special biopsy gun mechanism, is the most important factor for reducing bacterial translocation. Bacterial transfer may be further reduced by improved precision in manufacturing, material choice, or other surface treatments or coatings. The highly echogenic area on Tru-Cut needles has no use in TRbx because the biopsy guide on the ultrasound handle ensures that the needle is in focus and clearly visible.
We hypothesized that PEG coating would decrease bacterial adhesion to the needles, as PEG coating creates an inert surface by creating a steric barrier [Citation11], but our results do not support this hypothesis. A tentative explanation may be that, because the PEG brush only prevents surface adhesion forces operating on the nanoscale, it is unable to stop bacteria from being present on top of the coating. The roughness of the surface also plays a critical role since a PEG coating, tens of nanometres in thickness, cannot prevent bacteria form attaching in crevices on the metal surface that appear on the microscale. In repeat biopsy without cleaning the needle, which may better represent a prostate biopsy, the Forsvall needle also reduced bacterial load by 94%.
Strengths of this study include the use of human tissue to simulate a TRbx, the use of randomization and blinding during analysis to reduce bias, and consistent results for microscopy findings and bacterial culture analysis. There are, however, also some limitations. The patients preoperatively received antibiotics: Metronidazole with Trimethoprim/Sulfamethoxazole (TMP/SMX) or with Cefotaxime. TMP/SMX is sometimes used as prophylaxis in TRbx, but rarely Cefotaxime. The antibiotic resistance patterns of the bacterial flora in the colon tissue were not analysed and the effect of the prophylaxis was therefore not evaluated. On the other hand, the preoperative metronidazole should not change the interpretation of the results as anaerobes rarely cause infection after TRbx [Citation7]. Moreover, the properties of an ex-vivo colon may differ from those of an in-vivo colon, as the tissue may degrade and change its properties over time. The colon parts used may also differ from the properties of the rectum, particularly as the colon was stretched and thus slightly thinner than normal. Moreover, the colon specimens were cleared of all pericolic fat to ensure even thickness, but a TRbx needle typically passes through 2–5 mm of fatty tissue separating the colon and the prostate. Finally, the medium that we used was not solid, so it did not perfectly simulate a prostate biopsy. We hypothesise that, since almost all bacteria are trapped inside the Tru-Cut needle, the number of bacteria is not affected by its passage through periprostatic tissue. It will thus deliver its bacterial load into the prostate when the needle is opened to collect the specimen. Therefore, and due to the short exposure time, stirring and opening and closing the needle in the liquid medium after the puncture, would accurately capture the bacteria trapped in and on the needle.
We believe that the differences in bacterial load between the colon specimens were related to intra- and inter-patient variations in the colon contents, differences in the process of rinsing the colon mucosa, and possibly also by differences in bacterial antibiotic resistance between patients. Nonetheless, the results were consistent despite these possible differences.
In conclusion, we have shown that a smooth, forced-closed needle design significantly reduces bacterial transfer across the rectal wall in TRbx and is therefore likely to reduce the risk of infectious complications in TRbx. A clinical trial evaluating the quality of prostate specimens obtained by the Forsvall needle has been completed [Citation15] and its ability to reduce infectious complications will be studied further in a prospective, randomized multicentre trial.
Supplemental Material
Download PDF (244.7 KB)Acknowledgements
The authors thank Katarina Jandér for creating the illustrations, and Axel Ström for his assistance with the statistical analysis.
Disclosure statement
AF is listed as an inventor on patents for the novel (Forsvall) needle and is also the founder of Saga Surgical AB, a company owning the patent rights. All other authors have nothing to disclose and report no other potential conflicts of interest.
Additional information
Funding
References
- Liss MA, Ehdaie B, Loeb S, et al. An update of the American Urological Association White Paper on the prevention and treatment of the more common complications related to prostate biopsy. J Urol. 2017;198(2):329–334.
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64(6):876–892.
- Liss MA, Johnson JR, Porter SB, et al. Clinical and microbiological determinants of infection after transrectal prostate biopsy. Clin Infect Dis. 2015;60(7):979–987.
- Johansen TEB, Zahl PH, Baco E, et al. Antibiotic resistance, hospitalizations, and mortality related to prostate biopsy: first report from the Norwegian Patient Registry. World J Urol. 2020;38(1):17–26..
- Liss MA, Taylor SA, Batura D, et al. Fluoroquinolone resistant rectal colonization predicts risk of infectious complications after transrectal prostate biopsy. J Urol. 2014;192(6):1673–1678.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79(2):243–262.
- Williamson DA, Barrett LK, Rogers BA, et al. Infectious complications following transrectal ultrasound-guided prostate biopsy: new challenges in the era of multidrug-resistant Escherichia coli. Clin Infect Dis. 2013;57(2):267–274.
- Raman JD, Lehman KK, Dewan K, et al. Povidone iodine rectal preparation at time of prostate needle biopsy is a simple and reproducible means to reduce risk of procedural infection. J Vis Exp. 2015;(103):52670.
- Knop K, Hoogenboom R, Fischer D, et al. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49(36):6288–6308.
- Song DY, Herfarth KK, Uhl M, et al. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. Int J Radiat Oncol Biol Phys. 2013;87(1):81–87.
- Emilsson G, Schoch RL, Feuz L, et al. Strongly stretched protein resistant poly(ethylene glycol) brushes prepared by grafting-to. ACS Appl Mater Interfaces. 2015;7(14):7505–7515.
- Klar E, Rahmanian PB, Bucker A, et al. Acute mesenteric ischemia: a vascular emergency. Dtsch Arztebl Int. 2012; 109(14):249–256.
- Goldman E, Green LH. Practical handbook of microbiology. Boca Raton: CRC Press; 2009.
- Pilatz A, Veeratterapillay R, Koves B, et al. Update on strategies to reduce infectious complications after prostate biopsy. Eur Urol Focus. 2019;5(1):20–28.
- Forsvall A, Fisher J, Wagenius M, et al. Prostate biopsy quality and patient experience with the novel Forsvall biopsy needle – a randomized controlled non-inferiority trial. Scandi J Urol. 2021;55(3):235–241.
