Abstract
Background
Transrectal prostate biopsy (TRbx) carries an increasing risk of infection. The Forsvall Needle Prototype (FNP) is a novel biopsy needle that reduces bacterial load brought across the rectum and may therefore reduce infection risk. The objective of this study was to compare biopsy length, quality and patient experience for the FNP Version 2 (FNP2) versus a standard Tru-Cut needle.
Methods
We conducted a randomized, parallel-group, non-inferiority trial with twenty consecutive patients eligible for TRbx. Participants were randomized to undergo TRbx using either FNP2 or a standard Tru-Cut needle. The primary outcome was difference in mean biopsy lengths measured by the pathologist. FNP2 biopsy lengths ≤1.35 mm of the standard needle length were considered non-inferior. Secondary outcomes were biopsy length in the needle chamber and immediately after removal, biopsy quality, biopsy fragmentation, patient discomfort/pain, and complications (immediate and after 14 and 30 days).
Results
Mean pathologist-measured FNP2 biopsy length was non-inferior compared to the standard Tru-Cut needle (0.02 mm longer, 95%CI–0.73 to 0.76 mm). Biopsy length in the needle chamber and immediately after removal were also non-inferior. Biopsy quality and patient discomfort were not significantly different for the FNP2 and the standard Tru-Cut needle. Biopsy fragmentation was more common in the FNP2 group.
Conclusions
The FNP2 biopsy needle is non-inferior to the Tru-Cut needle in terms of biopsy length and not significantly different in terms of biopsy quality and patient experience. Future studies will evaluate the Forsvall needle design’s effect on post-biopsy infection risk.
Background
Prostate cancer is the second most common cancer in males with 1.28 million new cases in 2018 [Citation1]. It is mainly diagnosed and often monitored using tissue obtained by a Tru-Cut biopsy needle [Citation2]. Transrectal prostate biopsy (TRbx) is by far the most common biopsy method [Citation2]. In TRbx, the biopsy needle transfers colonic bacteria through the rectal wall into the prostate and periprostatic tissue, which may cause infectious complications including sepsis [Citation2–4].
Post-biopsy infection currently occurs in 2–10% of patients after TRbx [Citation2,Citation5]. At our institution, post-biopsy infections were found to occur at a rate of 5.4% [Citation6]. Antibiotic prophylaxis, which is routinely used to reduce the risk of post-biopsy infection, is becoming less effective as antimicrobial resistance continues to rise [Citation2,Citation7]. Previous work from our research group showed that a new biopsy needle design, the Forsvall needle prototype (FNP), reduced bacterial translocation across the rectal wall by 96%, and could therefore potentially reduce infectious complications following TRbx [Citation8].
However, even if the FNP is potentially safer needle, it must also obtain high-quality biopsy specimens and not increase the risk of other complications. In this study we used a prototype 2 version of the FNP (FNP2) designed to ensure biopsy quality () with maintained features that reduce bacterial transfer. We hypothesized that the FNP2 would be non-inferior to a standard Tru-Cut needle for TRbx in terms of biopsy length and not significantly different in terms of biopsy quality, patient experience, and complications.
Figure 1. Sketches and photos of the needles used in this study. (A) The Forsvall Needle Prototype 2 (FNP2) in the closed (top) and opened (bottom) positions. (B) A standard Tru-Cut needle in the closed (top) and opened (bottom) positions. Red arrow indicates a gap between the inner and outer needles that traps bacteria as it passes the colon wall, as shown in a previous study [Citation8]. (C) Photo of the Forsvall Needle Prototype 2 (FNP2) in the closed (top) and opened (bottom) positions. Scale bar indicates 10 mm.
![Figure 1. Sketches and photos of the needles used in this study. (A) The Forsvall Needle Prototype 2 (FNP2) in the closed (top) and opened (bottom) positions. (B) A standard Tru-Cut needle in the closed (top) and opened (bottom) positions. Red arrow indicates a gap between the inner and outer needles that traps bacteria as it passes the colon wall, as shown in a previous study [Citation8]. (C) Photo of the Forsvall Needle Prototype 2 (FNP2) in the closed (top) and opened (bottom) positions. Scale bar indicates 10 mm.](/cms/asset/4370ed0b-54fa-4943-a85a-78586ddd8ef5/isju_a_1921024_f0001_c.jpg)
Material and methods
Trial design
The study design was a randomized, controlled, parallel-group, non-inferiority trial with two arms. Allocation ratio was 1:1. The study was conducted as approved by the Regional Ethical Review Board at Lund University (registration number 2018/283). The trial was registered on clinicaltrials.gov with trial number NCT04091230 on September 16, 2019.
Participants
Patients were enrolled by a study nurse at Helsingborg hospital in September 2019. Eligibility criteria were indication for TRbx and written consent. The only exclusion criterion was unwillingness to participate.
Interventions
The Forsvall needle prototype (FNP) has a closed tip and a smooth outer surface when the needle is closed, which reduces the bacterial transfer from the rectum to the prostate and periprostatic tissues [Citation8]. The Forsvall Needle Prototype version 2 (FNP2) used in this trial maintains these infection preventing features, but the needle tip design and biopsy gun function was improved to ensure biopsy quality (). The FNP2 needle is used with a specific FNP2 biopsy gun (together referred to as the FNP2 system), and is operated in the same way as a standard Tru-Cut needle and biopsy gun.
The participants underwent TRbx with either the standard Tru-Cut needle normally used in Helsingborg hospital (Mermaid Medical M-biopsy Tru-Cut needle with Möller Medical Blue biopsy gun) or the FNP2 system. Both the standard and FNP2 needles were 18 Gauge (1.27 mm diameter), 25 cm long, and has a 19 mm sample notch (standard size for TRbx). The patients were placed in standard left side position and first had a digital rectal examination and a transrectal ultrasound. The prostate was anesthetized by 10 ml of 10 mg/ml mepivacaine evenly distributed between the base and apex of the prostate bilaterally using a standard 22 Gauge injection needle (Mediplast, Special cannula 0.7 × 200 mm). Nine to sixteen biopsy cores were then taken, according to standard procedures. All biopsies were taken by the same urologist (AF) and assisting nurse.
Randomization, allocation concealment, and implementation
After digital rectal examination and transrectal ultrasound, patients were randomized to TRbx using either the standard Tru-Cut needle or the FNP2. The assigned intervention group for the patients was printed on pieces of paper that were kept in closed, opaque, envelopes that were randomly mixed by shuffling. The next envelope in the stack was drawn by the study nurse and the participant was then assigned to the intervention group detailed in the envelope.
Blinding
Because of the obvious visual differences between the standard Tru-Cut needle and the FNP2, the involved urologist and study nurse could not be blinded to the type of needle used for each individual participant. The participants were blinded to the assigned intervention by keeping all equipment behind their back, so they could not identify which needle was used. Also the pathologists examining the biopsy specimens were blinded to the needle type used.
Outcomes
The primary outcome was biopsy core length, as measured by the pathologist after formalin fixation. Secondary outcomes were biopsy core length measured while the tissue core was in the needle chamber and immediately after removal, biopsy quality, biopsy fragmentation and patient reported discomfort and pain. Additionally, complications (immediate and after 14 and 30 days), which were not originally registered in the protocol on clincaltrials.gov, were added as planned secondary outcomes before the study was carried out.
Biopsy length was measured in three stages. First, following each biopsy, the needle was opened and the length of the biopsy was measured in the biopsy chamber by the study nurse with a standard ruler. The biopsy was then transferred to a transport paper and measured again with the ruler. The biopsy specimens were then placed in separate jars with formalin and sent to the pathology department, where they were prepared according to standard clinical practice. Samples were marked with a coded study label so that the pathology department was blinded to which biopsy needle was used. The biopsies were embedded in paraffin, and sectioned and mounted on glass slides. Images of the entire biopsy were obtained and biopsy length was measured using the measurement tool in computer program IDS7 (Sectra AB, Sweden) ().
In addition to the standard pathology report, the quality of the specimens was evaluated. The pathologists were asked to consider biopsy core length, thickness, fragmentation and artefacts, and use their experience from prior biopsies to score the overall quality of each biopsy core from 0 to 3, (0= inconclusive tissue; 1= poor; 2= good; 3= very good). Each specimen was first separately scored by two urology-focused pathologists, who then convened and agreed on a single score for each biopsy core.
The study participants were asked by the nurse to evaluate discomfort and pain using a numerical rating scale from 0 to 10, where 0 represented no discomfort or pain and 10 represented the worst discomfort or pain imaginable. The numerical rating scale was used as it is a sensitive measure of pain [Citation9]. Participants were asked to evaluate discomfort and pain at A) probe placement in the rectum (before randomization), B) upon injection of the mepivacaine at the base and apex (average of left and right sides) before randomization and C) at biopsy sampling (average for all biopsies taken). Because pain reporting is a subjective measure, we included pain scores at A and B as a reference in case of baseline differences in pain reporting between the two groups.
Any technical problems or immediate complications were registered at the time of biopsy. The patients were asked to report any complications (bleeding, infection symptoms, other symptoms) when returning for the results of the biopsy around day 14 (14-day complications) and again by phone and retrospective review of patient records at day 30 (30-day complications). The assigned intervention was not revealed to participants until the end of the follow up period (30 days).
Sample size
As a test sample, lengths of biopsies taken using a standard Tru-Cut needle from 20 consecutive patients biopsied by the author AF from January to March 2019 at Helsingborg Hospital were obtained from pathology reports. Their mean length was 13.5 mm. A previous study suggested that a biopsy length of 13.5 ± 3.2 mm is ideal for Gleason grading (Reis 2014). Therefore, when calculating the required sample size, we chose a non-inferiority limit well within this range: 10% of the average length (1.35 mm). We expected an average of 12 biopsy cores per patient, and that biopsy cores were the unit of analysis for the primary outcome. A sample size calculation showed that 116 biopsies were needed in each group to achieve 90% power with α = 5% and a 95% CI of −1.35 mm, which corresponds to 10 patients in each group.
Statistical analysis
Distribution of continuous data (biopsy length) was determined by visual examination of histograms to be sufficiently close to a normal distribution, and therefore parametric tests were used. Variances were compared using Levene’s test and were found to be equal for all measurements. We calculated 95% confidence intervals (CIs) for the difference between the mean biopsy core lengths in the FNP2 group versus in the standard biopsy needle group, and we only considered the lower bound of the 95% CI when assessing non-inferiority. The non-inferiority margin was set as a mean difference of −1.35 mm, i.e. 10% shorter than the mean standard needle biopsy length in the test sample, described above. We pre-specified that non-inferiority would be concluded if the 95% CI of the pathologist-measured difference was over −1.35 mm, i.e. that the lower 95%CI for the FNP2 biopsy core length is at most 1.35 mm shorter than standard biopsy core mean.
Categorical data (biopsy quality and fragmentation, discomfort/pain, and complications were reported as medians with interquartile ranges (IQR) and groups were compared using the Mann-Whitney test. P-values below 0.05 were considered statistically significant.
Results
Participants
A total of 20 patients were enrolled and randomly allocated to the two groups, with 10 patients in each group (). The patients in the FNP2 group were older, but otherwise the clinical characteristics were similar in the 2 groups ().
Table 1. Clinical characteristics of the participating patients.
All patients completed the assigned intervention and none was lost to follow-up. Data for biopsy length after removal from the chamber was missing from one patient in each group.
Biopsy length and quality
In total, 119 biopsies were taken with the prototype system and 130 with the standard needle. The mean biopsy core length was 0.016 mm longer (95%CI −0.73 to 0.76 mm) in the FNP2 group than in the standard needle group, when measured by the pathologist ( and ). When measured in the chamber and after removal from the chamber, FNP2 biopsies were 0.26 mm (95%CI −0.94 to 0.42 mm) and 0.03 mm (95%CI −0.70 to 0.64 mm) shorter than the standard biopsies. All lower 95% CIs were within the pre-specified non-inferiority limit of −1.35 mm, allowing for the conclusion that the FNP2 is non-inferior to the standard needle. Representative images of biopsy core sections taken by the FNP2 and standard Tru-Cut needle are shown in .
Figure 3. Biopsy length, biopsy quality, and patient experience using the FNP2 and standard tru-cut needle. (A) Difference between mean biopsy length of biopsies taken by the FNP2 and standard tru-cut needles. The symbols represent means and whiskers represent 95% confidence intervals. Dashed line is the pre-determined non-inferiority margin (mean difference 1.35 mm shorter than the standard tru-cut needle biopsy length). (B–G) Histograms showing the distribution of biopsy quality scores taken by (B) a standard tru-cut needle and (C) the FNP2, the distribution of biopsy fragments in cores taken by (D) a standard tru-cut needle and (E) the FNP2, and the distribution of pain/discomfort scores for biopsy taken by (F) a standard tru-cut needle and (G) the FNP2.
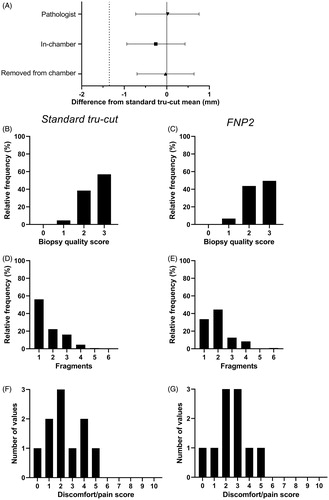
Figure 4. Representative images of biopsies taken by standard Tru-cut and FNP2 needles, with and without fragmentation. Black lines show the digital measurement path and grey boxes are annotations indicating the measured length of each fragment.
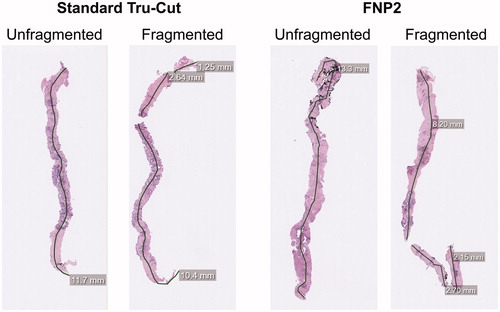
Table 2. Biopsy length and biopsy quality of biopsy cores taken by a standard Tru-Cut needle and the FNP2 and patient experience of the procedure.
Biopsy quality was not significantly different (p = 0.10), with a median score of 2 (IQR 2–3) for the FNP2 and 3 (IQR 2–3) for the standard needle (), and histograms indicated a similar distribution of quality scores for each needle (). Biopsy fragmentation was significantly different (p = 0.007), with a median of 2 (IQR 1–2) fragments obtained by the FNP2 and median 1 (IQR 1–2) fragment obtained by the standard needle (, ).
Patient experience
There was no significant difference in median discomfort/pain during injection of the local anesthetic or during biopsy, but pre-randomization placement of the TRUS caused more discomfort/pain in the group later randomized to the FNP2 (). The distribution of scores during biopsy was similar for both needles ().
There were no mechanical problems or immediate complications with either needle, other than expected minor self-limiting urinary or rectal bleeding. One patient in the standard needle group was hospitalized with post biopsy sepsis 48 h following biopsy. No infections occurred in the FNP2 group. No other complications were reported at the 14- or 30-day follow-ups ().
Table 3. Complications and results of biopsy.
Discussion
This is the first trial in humans with the novel Forsvall biopsy system, consisting of the Forsvall needle prototype version 2 (FNP2) and a modified biopsy gun. FNP2 includes the features of the Forsvall prototype 1 with a closed tip and a smooth outer surface that has been shown to reduce bacterial transfer across colon tissue by 96% [Citation8]. We found that the FNP2 was non-inferior in terms of biopsy length when measured at three different points – in the chamber, immediately after removal from the chamber and by the pathologist. The FNP2 did not result in significant differences in biopsy quality or patient experience compared to a standard Tru-Cut needle, but it did result in more biopsy specimen fragmentation.
Fragmentation may be due to tissue properties, but can also occur during any step from obtaining the biopsy to the fixation process. The presence of cancer and increased Gleason score can lead to more biopsy fragmentation [Citation10], although we did not find more fragmentation in cancerous cores in this material. A reason for the differences in fragmentation may be the slightly larger head of the FNP2, which made it somewhat harder to remove the specimen from the FNP2 needle chamber when using the standard method of rolling the needle chamber on the transport paper. For future studies we will employ an alternative method for removing the biopsy from the chamber that may reduce the risk of fragmentation.
The biopsy core lengths obtained in our study were similar to these reported previously. Achieving adequate core length is crucial for the diagnosis of cancer. In two recent retrospective studies of over 2000 biopsies each, the average biopsy length was between 11.3 and 13.3 mm [Citation11,Citation12]. In a study of over 17,000 biopsies the average length was 10.44 ± 2.36 mm [Citation13]. Reis and co-workers found that, among patients undergoing radical prostatectomy, those presenting with underestimated Gleason score had a median core length of 11.4 mm while those with no change in Gleason score had a median core length of 13.7 mm [Citation14]. In contrast to these studies that measured length after fixation but before paraffin embedding, we report the length measured in computerized images of biopsies obtained after sectioning and mounting on glass slides, which could cause some differences in both length and shape. Nonetheless, the mean pathologist-measured length of biopsies taken by the FNP2 was 12.96 mm, which lies within the ranges reported in the previous studies.
Biopsy outcome was in line with the pre-biopsy clinical suspicion for both systems, with more clinically significant cancer being detected in the FNP2 group. As expected when using needles of the same length and diameter there was no difference in bleeding [Citation2], with 60–70% of patients describing a mild self-limiting transrectal or transurethral bleeding in both groups, none persisting at 14-day follow-up. Similarly, patient experience, measured by discomfort and pain score, was not significantly different between groups. We note that one patient in the standard Tru-Cut group suffered a post-biopsy sepsis, the very complication the FNP2 aims to reduce. The sample size was too small to draw conclusions about complication rates and patient experience and so these factors will be further studied in a large trial in the future.
Strengths of this study include the randomized design and the blinding of patients and pathologists to reduce bias. Additionally, to reduce effects of sample handling and bias, we made multiple length measurements at various stages (inside and outside of the chamber, and by the pathologist), and all biopsies were done by the same doctor/nurse team to reduce inter-operator variability. A limitation is that, due to differences between the instruments that were impossible to mask, the study nurse and urologist were not blind to the needle used. Additionally we did not conduct a questionnaire to assess the success of the blinding method for the participants after the biopsy, although the visual differences between the needles were so small that accidental un-blinding was very unlikely.
In conclusion, we found that the Forsvall needle prototype 2 is non-inferior to the standard Tru-Cut needle for prostate biopsy acquisition in terms of biopsy length, and not significantly different in terms of biopsy quality and patient experience. We will evaluate the Forsvall needle design in a multicenter randomized controlled trial to determine if it can reduce post biopsy infections.
Acknowledgement
The authors thank research nurse Annika Skoog, photographer Charlotte Carlberg-Bärg, and statistician Axel Ström.
Disclosure statement
Andreas Forsvall is listed as an inventor on patents for the new (Forsvall) needle system and is also the founder of Saga Surgical AB, a company owning the patent rights for this needle. Other authors have nothing to disclose.
Data availability statement
The FNP2 system is an investigational device and has to undergo FDA-approval and CE-labelling to be made available.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Liss MA, Ehdaie B, Loeb S, et al. An update of the american urological association white paper on the prevention and treatment of the more common complications related to prostate biopsy. J Urol. 2017;198(2):329–334.
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64(6):876–892.
- Liss MA, Johnson JR, Porter SB, et al. Clinical and microbiological determinants of infection after transrectal prostate biopsy. Clin Infect Dis. 2015;60(7):979–987.
- Johansen TEB, Zahl PH, Baco E, et al. Antibiotic resistance, hospitalizations, and mortality related to prostate biopsy: first report from the Norwegian Patient Registry. World J Urol. 2020;38(1):17–26.
- Forsvall A, Jönsson H, Wagenius M, et al. Rate and characteristics of infection after transrectal prostate biopsy – a retrospective observational study. Scand J Urol. 2021. doi:10.1080/21681805.2021.1933169.
- Liss MA, Taylor SA, Batura D, et al. Fluoroquinolone resistant rectal colonization predicts risk of infectious complications after transrectal prostate biopsy. J Urol. 2014;192(6):1673–1678.
- Forsvall A, Fisher J, Cardoso JFP, et al. Evaluation of the Forsvall biopsy needle in an ex vivo model of transrectal prostate biopsy - A novel needle design with the objective to reduce the risk of post-biopsy infection. Scand J Urol. 2021;55(3):227–234.
- Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404.
- Fajardo DA, Epstein JI. Fragmentation of prostatic needle biopsy cores containing adenocarcinoma: the role of specimen submission. BJU Int. 2010;105(2):172–175.
- Ergün M, İslamoğlu E, Yalçınkaya S, et al. Does length of prostate biopsy cores have an impact on diagnosis of prostate cancer? Turk J Urol. 2016;42(3):130–133.
- Fiset PO, Aprikian A, Brimo F. Length of prostate biopsy cores: does it impact cancer detection? Can J Urol. 2013;20(4):6848–6853.
- Yılmaz H, Yavuz U, Üstüner M, et al. Longer biopsy cores do not increase prostate cancer detection rate: A large-scale cohort study refuting cut-off values indicated in the literature. Turkish Journal of Urology. 2017;43(3):297–302.
- Reis LO, Sanches BC, de Mendonça GB, et al. Gleason underestimation is predicted by prostate biopsy core length. World J Urol. 2015;33(6):821–826.